Researchers have used a method of total-body imaging to measure and track the body’s immune response to viral infection. The method is a promising platform for studying human immunity in greater detail and could be used to evaluate cancer treatments and study other infectious and autoimmune diseases.
During a viral infection, non-customized (naïve) CD8+ T cells become activated and cytotoxic, seeking out and killing infected cells. Some CD8+ cells develop into pathogen-specific memory T cells, which ‘remember’ the virus, providing the immune system with long-term protection should it return.
Understanding how the immune system responds to viral infections and develops an invader-specific memory is important to developing vaccines and treatments. While CD8+ T cells can be found in the blood, they mostly reside in non-blood tissue such as the spleen, bone marrow and lymph nodes, so accessing them requires a tissue biopsy.
Now, researchers from UC Davis Health have developed a non-invasive method of measuring CD8+ T cells and their response to viral infections using total-body positron emission tomography (PET) scans.
“There has been a growing interest in studying the critical role of CD8+ T cells in immune response and memory,” said Negar Omidvari, the study’s lead author. “However, evaluating immunological changes in non-blood tissue is challenging due to the invasive nature of biopsies. In some cases, it is not even practice in certain anatomical regions of living participants, such as the brain, spinal cord, cardiopulmonary tissue and vascular tissue. So, the challenge was to find non-invasive quantitative methods for measuring CD8+ T cell distribution and trafficking in the body that are safe to also use in healthy people.”
Dynamic total-body PET involves injecting a very small amount of a radioisotope tracer into the patient, followed by continuous imaging over a period of time, which creates a movie showing the tracer’s kinetics (distribution over time) within the body. Then, mathematical models are used to extract biologically relevant information.
Total-body PET scanners enable simultaneous dynamic imaging and kinetic modeling across all organs. They’re more sensitive than conventional PET scanners, translating to better image quality and lower radiotracer injection doses. It’s the first time dynamic PET and kinetic modeling have been used to measure human CD8+ T cell distribution.
“Dynamic total-body PET is currently the only available technology with [an] acceptable radiation dose that allows non-invasive quantitative measurements of immune cell distribution and trafficking (movement) inside all tissues in living humans,” Omidvari said.
The researchers enrolled three healthy adults and five recovering from COVID-19 infection with mild-to-moderate symptoms not requiring hospitalization. Participants were injected with a small amount of radioactive liquid containing an immunoPET radiotracer, 89Zr-Df-Crefmirlimab, that targets human CD8 cells.
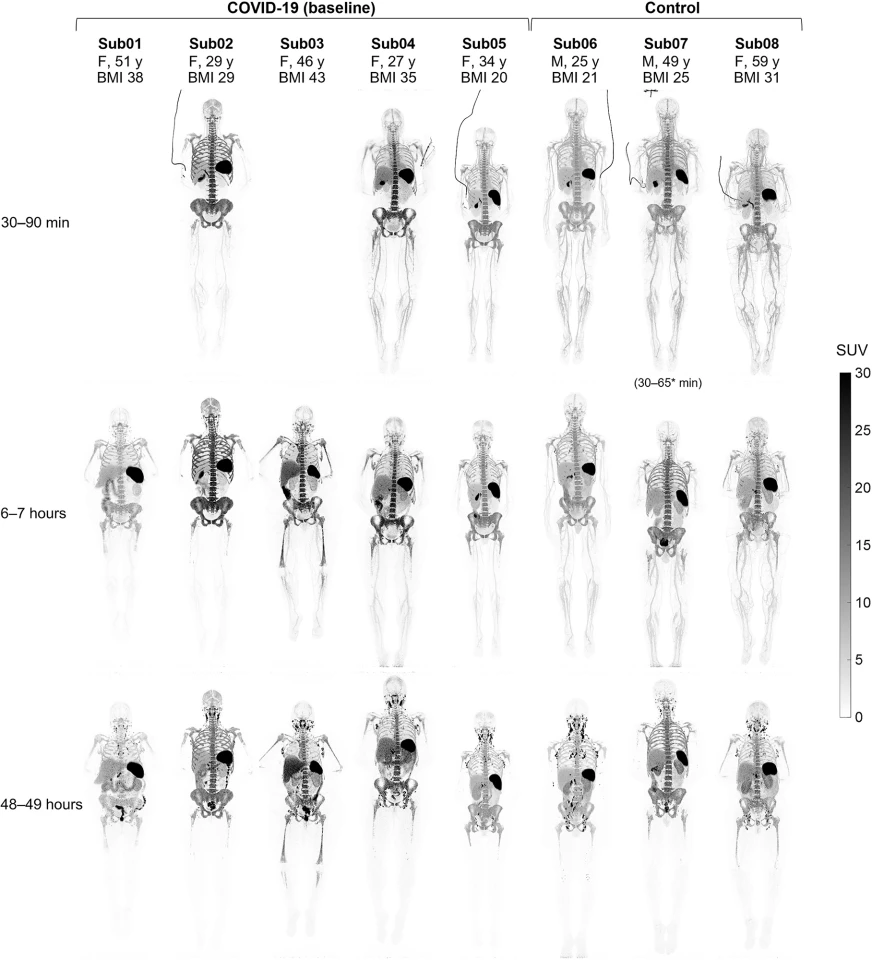
Each participant had a dynamic 90-minute scan, a 60-minute scan at six hours and a 60-minute scan 48 hours after injection with the radiotracer. Kinetic modeling allowed the researchers to separate the effect of blood circulation on tissue and measure the radiotracer uptake in tissues independent of when the imaging was done and differences in each participant’s blood.
The images showed a high uptake of CD8+ T cells in the lymphoid organs of all participants. The highest was in the spleen, followed by the bone marrow, liver, tonsils and lymph nodes. Significantly, the researchers observed increased concentrations of CD8+ T cells in the bone marrow of recovering COVID patients compared to the controls. Six months post-COVID infection, follow-up scans showed the concentration of memory T cells in recovering patients was slightly higher than scans taken at baseline in all bone marrow regions.
“Bone marrow has been identified as a major pool and the preferred site for proliferation of memory CD8+ T cells following a viral infection,” Omidvari said. “This trafficking of memory T cells to certain tissues like the bone marrow is critical to developing immune memory after viral infection.”
With their study, the researchers have provided a new platform for studying the human immune response and memory in all organs in a non-invasive way.
“What is fundamentally important about this study is that it showed the potential of total-body PET to assess T cell distributions across the entire human body, with the image quality necessary for detailed modeling, and with a radiation dose that is low enough to allow its broad application to study the immune response in humans,” said Simon Cherry, a co-author. “In our case, we were able to characterize the dynamics of this immunoPET tracer in healthy control subjects and in patients with infectious disease (COVID-19), which is an important first.”
In addition to studying immune response and memory, the researchers say the method could be used to evaluate treatment response in cancer patients and may extend to studies of infectious and autoimmune diseases and organ transplantation.
The study was published in the journal Science Advances.
Source: UC Davis Health







