Researchers have created a slow-release ‘smart’ insulin that responds to blood glucose levels to provide week-long control with virtually no incidents of low blood sugar. Tested in mice and minipigs, the novel insulin opens the door to once-a-week insulin injections for diabetics.
One of the (literal and figurative) pains for a diabetic is the need for multiple daily insulin injections. Insulin replacement therapy aims to maintain normal blood glucose (BG) levels, with diabetics often walking a fine line between hypoglycemia (low BG) and hyperglycemia (high BG), both of which can present serious health problems. While it’s an effective regime, combining a long-acting ‘basal’ insulin with boluses of fast-acting insulin taken with meals can be burdensome because of the number of injections it requires.
From developing a subcutaneous implant containing insulin-secreting cells or a hydrogel infused with slow-release glucagon-like peptide (GLP-1), researchers have long focused on reducing the burden of multiple insulin injections on diabetics. Now, researchers from the College of Pharmaceutical Sciences at Zhejiang University, China, have developed an injectable ‘smart’ insulin injection that lasts for a week – or longer – and is responsive to BG.
“This is an exciting breakthrough in the development of next-generation insulin,” said John Buse, one of the study’s co-authors. “In the past decades, many researchers are trying to make insulin usage convenient and safe.”
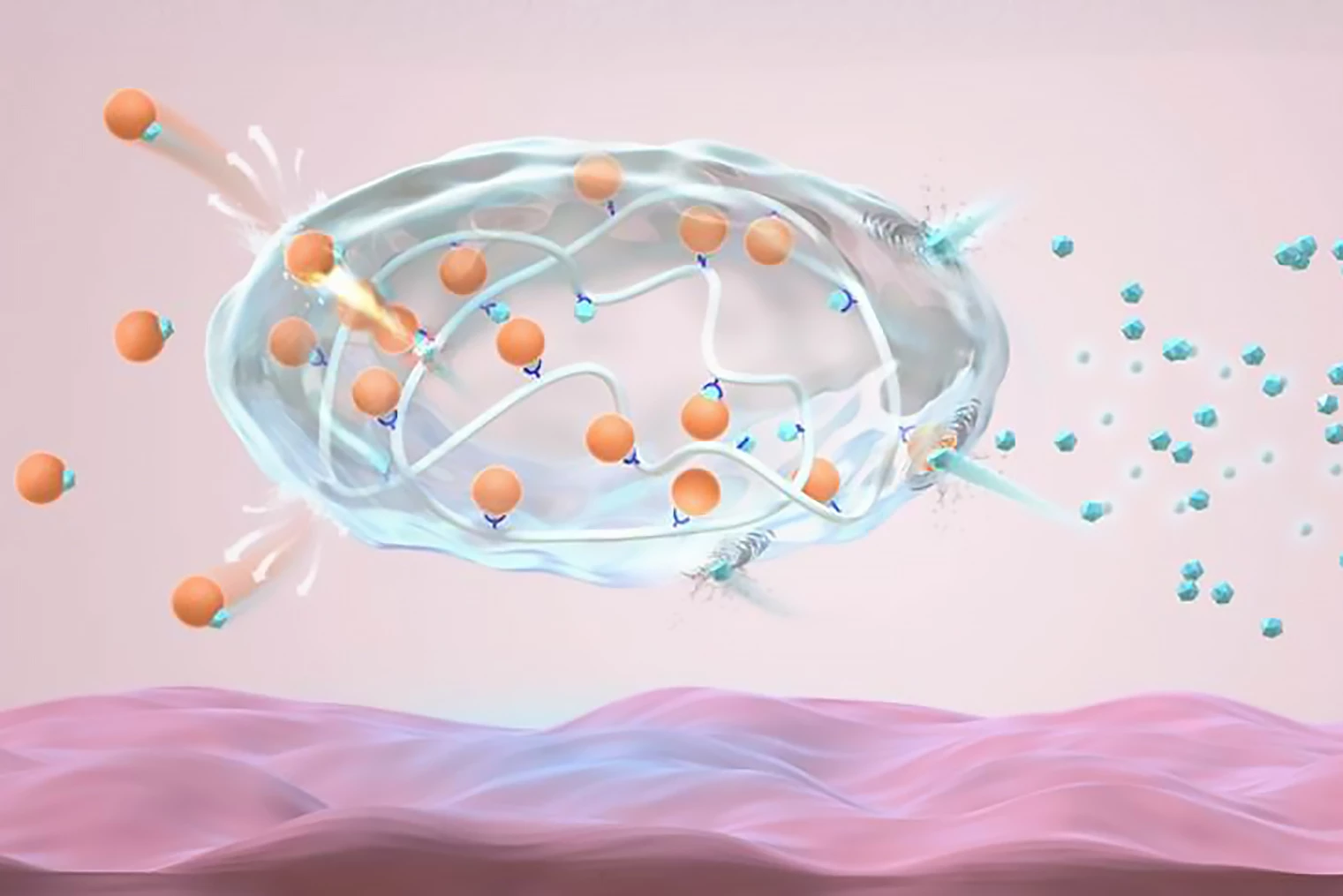
The researchers created an analog of recombinant human insulin, the insulin used in current injections, by modifying it with gluconic acid. Called Glu-insulin, it forms a stable complex with 4-carboxy-3-fluorobenzeneboronic acid (FPBA)-modified poly-L-lysine, a polymer, through dynamic electrostatic attraction and complexation, which, in simple terms, is a chemical reaction that forms a complex compound.
When BG is normal (normoglycemia), both the strong electrostatic force and the complexation cause the formulation to release an ultra-small, slow and continuous amount of insulin. In hyperglycemic conditions, glucose binds to the FPBA and reduces the electrostatic attractions and density of the bonds between Glu-insulin and the polymers, promoting insulin release.
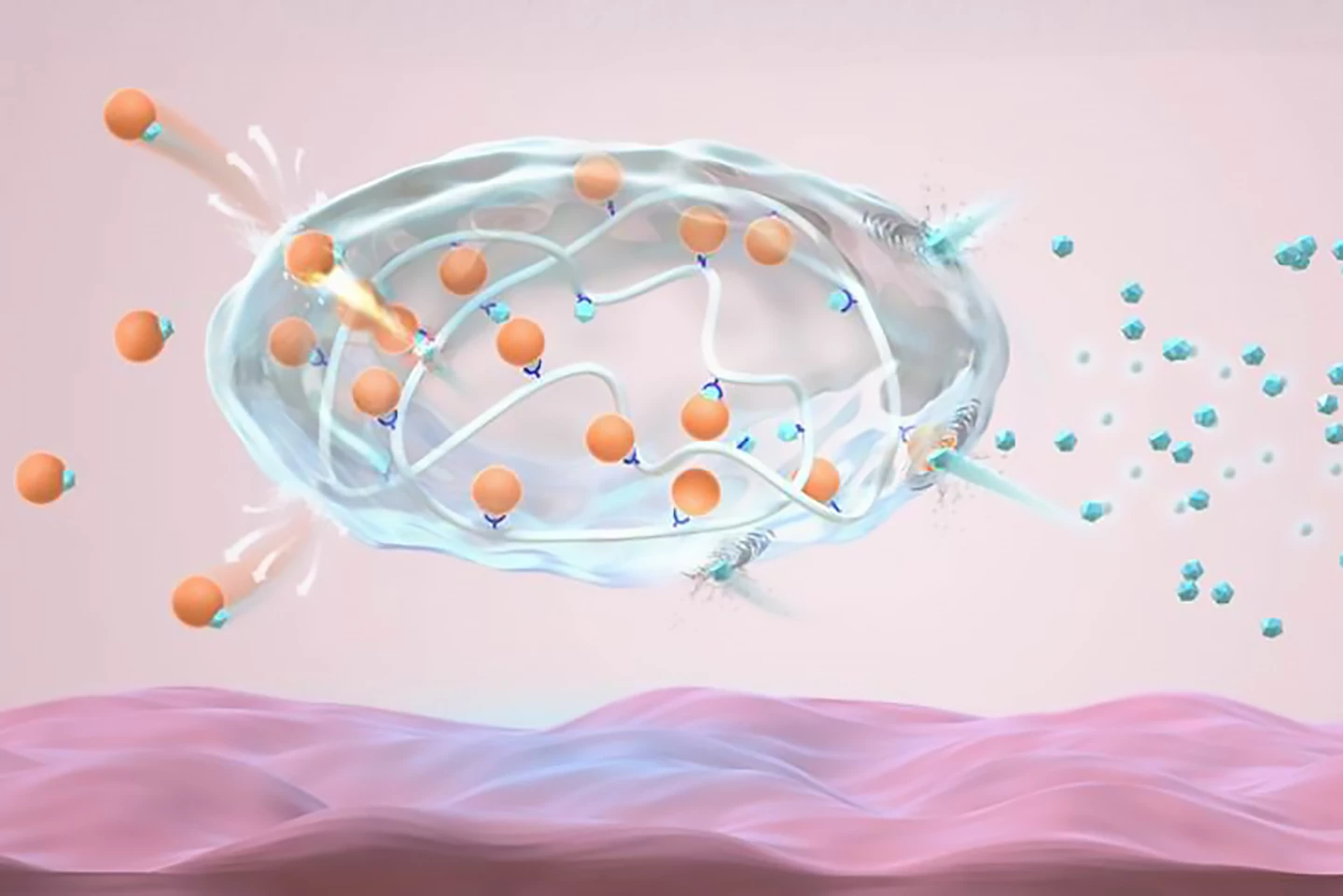
Tested in a type 1 diabetic mouse model, the injected novel insulin complex formed a pea-sized depot – a slow-release form of medication – under the skin, maintaining blood insulin levels at a steady ‘basal’ level and BG levels within a normal range. The researchers found that injecting the mice with glucose, which elevated BG, triggered insulin release to correct the raised glucose within two hours. As the BG returned to normal, it gave the complex a negative feedback signal to reduce the insulin release rate. Importantly, the novel depot resulted in “negligible” hypoglycemia.
The insulin complex achieved normoglycemia for a week following administration and did not create a fibrous capsule, which can occur with depot injections when the host’s immune system reacts to the foreign body and can interfere with drug infusion. The same results were achieved when the insulin depot was injected into minipigs.
“Current study in the minipig model indicate that this new formulation has long-lasting and glucose-responsive insulin release properties,” said Zhen Gu, corresponding author. “It could maintain normal blood sugar levels in 30 kg (66 lb) minipig model for over one week with a single dose, without symptoms of hypoglycemia."
The researchers plan to further assess the long-term biocompatibility of this formulation before commencing clinical trials.
The study was published in the journal Nature Biomedical Engineering.
Source: Zhejiang University