When you get right down to the atomic scale, things happen much faster than we’re used to. That makes it hard to actually see what happens during chemical reactions, so researchers at Harvard have found a way to slow things down. By cooling molecules to a fraction above absolute zero, the team was able to perform the coldest chemical reaction ever recorded, capturing a never-before-seen action as two molecules swapped atoms.
Absolute zero – which is -273.15° C or -459.67° F – is regarded as the coldest possible temperature. At that point, molecules essentially stand still, resulting in a complete absence of motion and heat.
For the new study, the Harvard team chilled molecules down to a few millionths of a degree above absolute zero – a temperature of 500 nanoKelvin. That’s way colder than any naturally occurring temperature in the universe. For comparison, at its coldest interstellar space only gets down to a balmy 3 Kelvin, which is 3 billion nanoKelvin.
It’s not the coldest unnatural place though – that honor still belongs to the Cold Atom Lab aboard the International Space Station, where temperatures have been made to plunge to just 100 nanoKelvin.
In the Harvard study, the researchers worked with a gas made up of potassium and rubidium atoms. When these molecules collide they swap partners, creating a new molecule of two potassium atoms and another with two rubidium atoms.
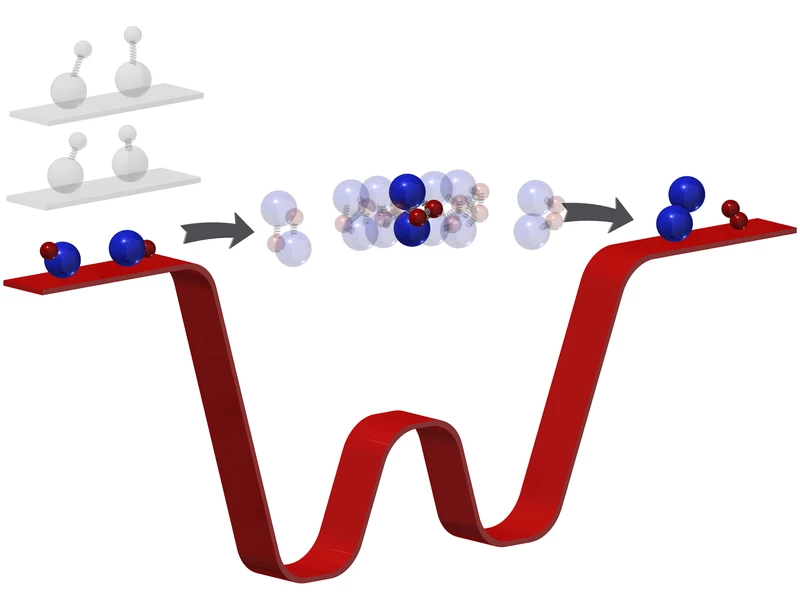
Normally, chemical reactions between molecules happens far too fast for scientists to see everything – even the most precise equipment can only watch the original molecules disappearing and the new ones appearing in their place. The middle step remained a mystery.
But now, the researchers have seen that missing link. At this ultracold temperature, the chemical reaction takes place millions of times slower, giving the team a wider window to watch what’s going on.
When the potassium-rubidium molecules collided, the team was able to image for the first time the four-atom molecule that’s briefly created as an intermediate step. That allows the team to watch atomic bonds break and new ones form.
With this ability, the researchers say that in future they could study chemical reactions in much more detail. As well as just watching, the extended window could allow scientists to intervene in chemical reactions more precisely, which in turn may lead to a whole range of new applications. After all, chemical reactions are at the heart of pharmaceuticals, energy and household products, just to name a few things.
The research was published in the journal Science.
Source: Harvard




