A new study into the biological mechanisms underlying depression may have upended the widely held belief that the condition is associated with inflammation. It found that suppression of the brain’s immune cells might be a contributing factor instead.
Depression is a leading cause of disability worldwide. Up to 30% of people with major depressive disorder (MDD) have conditions that are resistant to treatment, driving a need for insight into what causes the debilitating condition and the development of new therapies to treat it.
Inflammation has long been linked to depression, based on studies that have found pro- and anti-inflammatory proteins (cytokines and chemokines) in the blood and post-mortem brain tissue of people with MDD. Furthermore, other studies have shown a higher prevalence of MDD in people with chronic inflammatory diseases like rheumatoid arthritis, inflammatory bowel disease and multiple sclerosis. And inflammation is known to affect microglia activity.
Microglia are the brain’s immune cells, making up 10% to 15% of cells in the brain. In healthy brains, microglia are extremely dynamic, constantly moving around the brain to ensure its tissues function correctly. They clear out cellular debris and excess proteins, maintain neuronal networks, and repair injuries.
Research has found microglial abnormalities in psychiatric disorders such as schizophrenia, autism spectrum disorder and bipolar disorder. But research into microglia in people with MDD has been contradictory; science is uncertain whether microglia have positive or negative effects.
This uncertainty prompted researchers from the Netherlands Institute for Neuroscience to look more closely at microglia – and inflammation – and their role in depression.
The researchers examined the white and gray matter in post-mortem human brain tissue taken from 13 people with depression. They compared these with samples from 10 control donors.
“During the study, we used fresh tissue immediately after death to isolate microglia and compare these between depressed people and controls,” said Karel Scheepstra, the study’s lead author. “We saw abnormal microglia in depressed patients, with the greatest abnormalities seen in patients who were most depressed just before death.”
The researchers found inactive microglial only in the gray matter. Gray matter contains a large number of neurons that allow it to process information and control movement, memory and emotion.
“This suggests that there is a likely interaction between the microglia and the structures located in the gray matter: the neurons and synapses,” Scheepstra said.
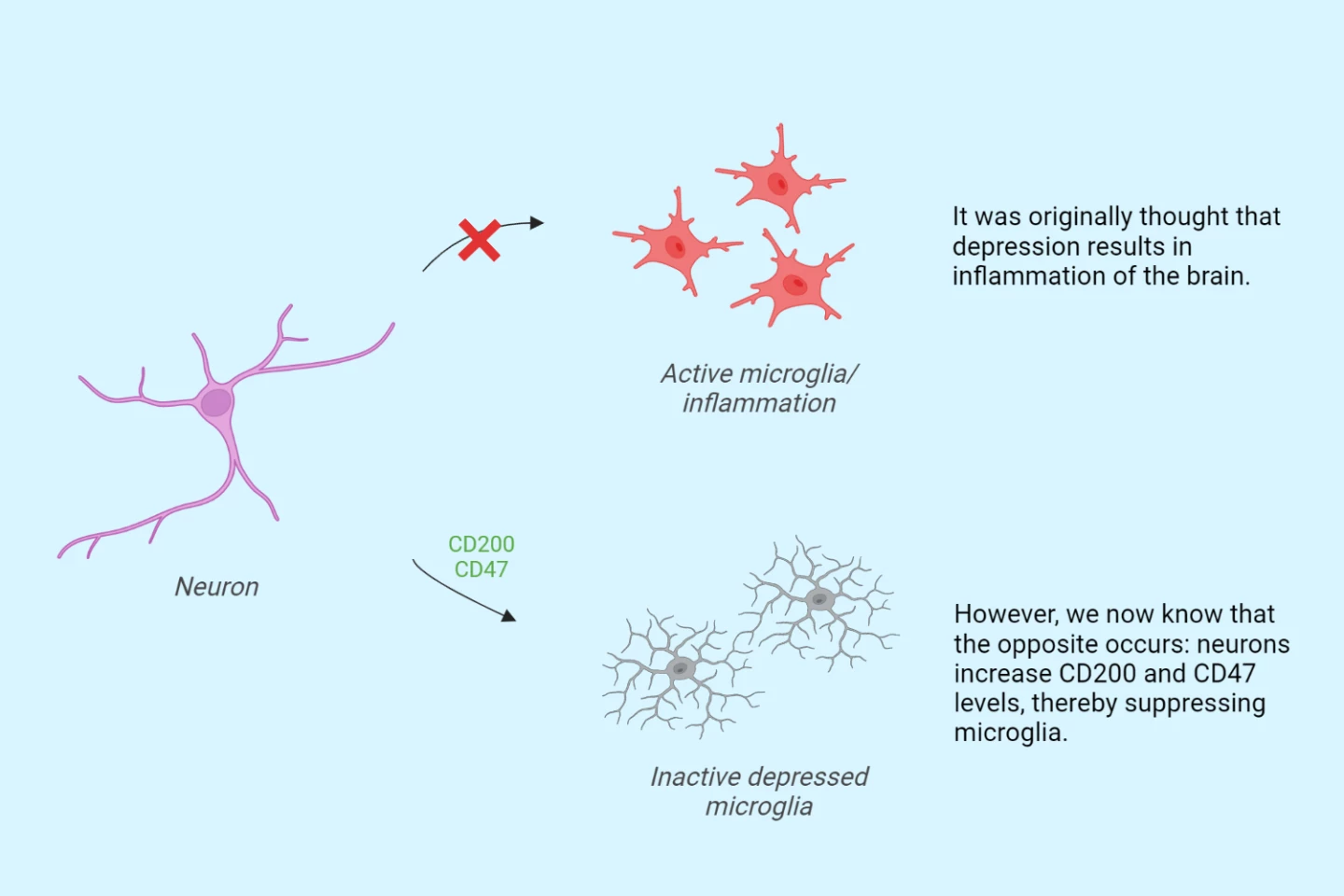
Wanting to understand whether, and how, the inactive microglia contributed to depression, the researchers performed RNA sequencing to check for gene expression changes. They found two proteins, CD200 and CD47, present in elevated levels in the gray matter neurons and synapses. Both are known to silence microglial immune responses.
Their findings upend the widely held belief that depression is associated with inflammation.
“We’ve hypothesized for years that depression is associated with inflammation of the brain, but we’re now seeing just the opposite: not neuroinflammation, but rather an immune-suppressed type of microglia,” said Scheepstra. “We termed them ‘depressed microglia’ and we wondered how exactly this is possible. The proteins CD200 and CD47 are located on brain cells and synapses. They interact with microglia and are, as it were, a kind of ‘don’t eat me’ signal. What we saw is that these proteins were elevated, resulting in suppressed microglia, thereby possibly preventing them from clearing damaged connections.”
The researchers say this might explain why trials using anti-inflammatories to treat MDD have failed. They also point out that the samples they used came from late-stage MDD cases, and it could well be that inflammation and microglial activation occur in earlier stages of the condition.
The next step for the researchers is to see how the inactive microglia affect the maintenance and formation of connections between neurons. And it may pave the way for novel therapies.
“If we know where things go wrong in the process, this can provide targets for new medication,” Scheepstra said. “Can we make these microglia more active again? And what effect does this have on the course of the disease? For now, we have shown that the brains of people who were depressed during life show altered cell activity. This gives us a better understanding of what goes wrong, which we can then build on.”
In the meantime, ways to maximize the health of your microglia include eating fruit, vegetables, and healthy fats. A good gut microbiome has a positive effect on the development, maintenance and overall health of microglia. And avoid alcohol and smoking, which can activate the microglial inflammatory response, as does lack of sleep.
The study was published in the journal Biological Psychiatry.





