Implants and tiny machines could eventually be working inside our bodies to help treat disease or monitor activity, but letting them communicate is tricky. Now scientists at EPFL have developed a system whereby devices can communicate by releasing molecules into a patient’s bloodstream.
Biomedical implants play a key role in healthcare, monitoring activity in organs like the heart or brain, while recent research is investigating how nanoscale robots might one day swim or crawl through the body to fight disease. But these systems have a communication issue.
Running wires through the body is not only impractical, it’s an infection risk. And wireless technologies like radio, light and Bluetooth don’t travel through human tissue very efficiently, drastically limiting their range.
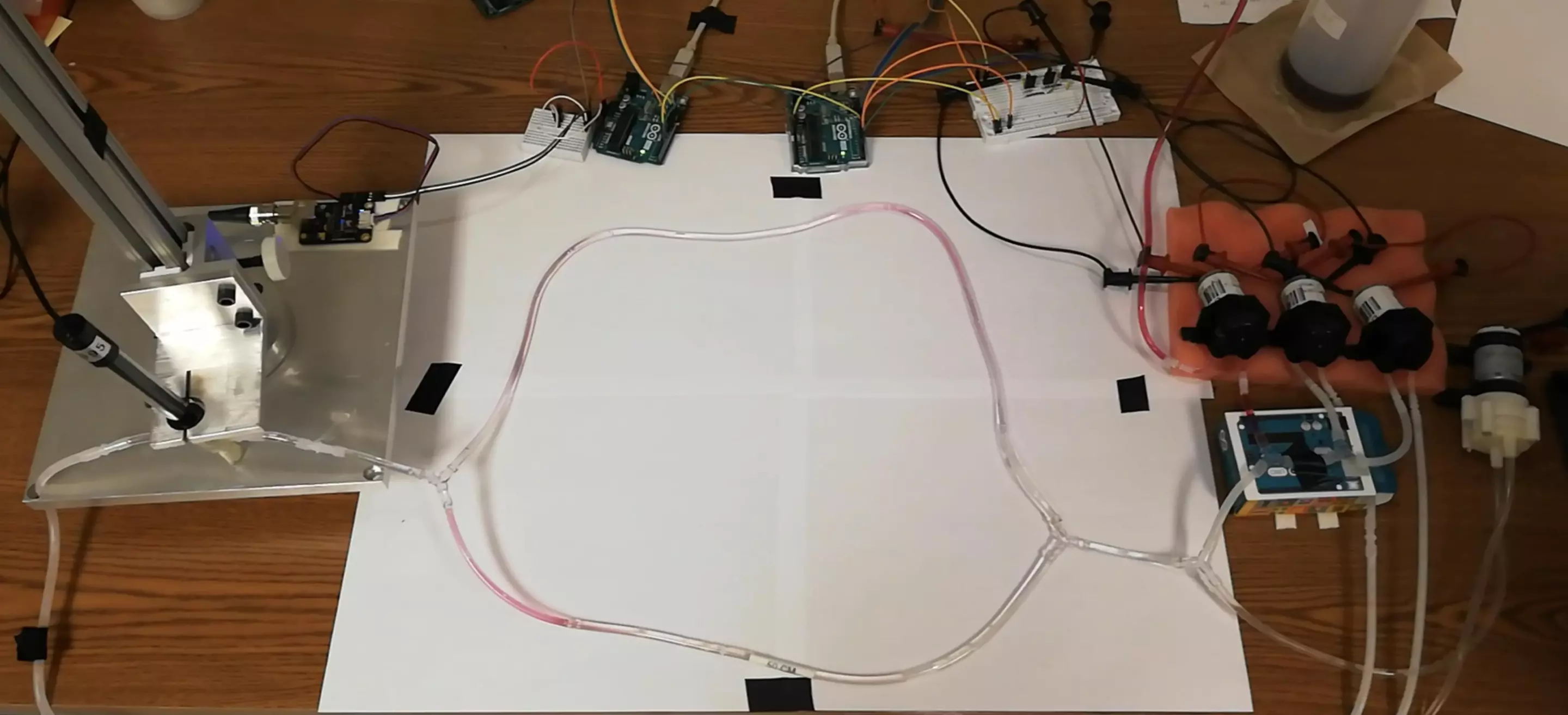
Now, scientists at EPFL have demonstrated a proof-of-concept system called biomolecular communication. The idea is to allow micro- or nano-robots and implants to communicate by releasing specific molecules into the bloodstream – in a basic sense, the presence of a molecule could be interpreted by a machine as a 1, while no detection represents a 0.
“Biomolecular communication has emerged as the most suitable paradigm for networking nano-implants,” said Haitham Al Hassanieh, an author of the study. “It’s an incredible idea that we can send data by encoding it into molecules which then go through the bloodstream and we can communicate with them, guiding them on where to go and when to release their treatments, just like hormones.”
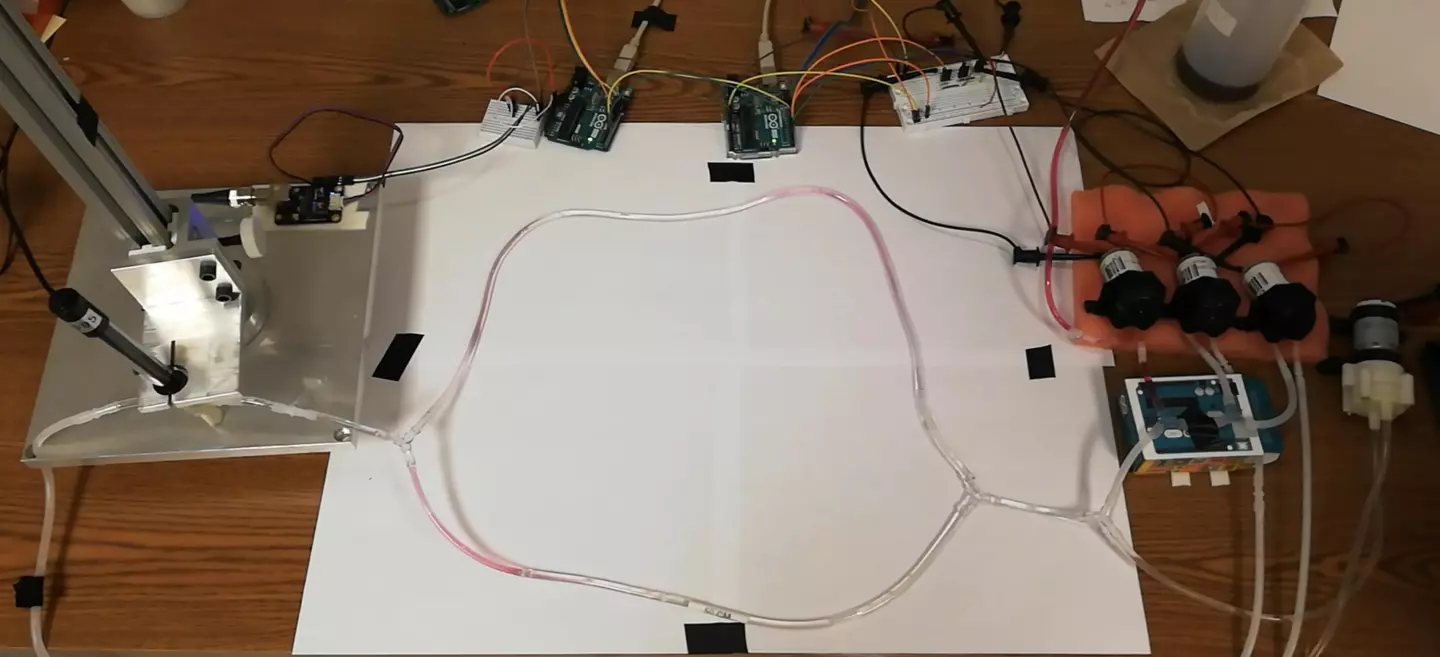
The team translated techniques from electronic networking, such as packet detection, channel estimation, and encoding and decoding schemes, to the molecular network. This helped to overcome problems raised by biology, like unstable channels and a lack of synchronization and feedback.
The researchers tested the technology on a synthetic circulatory system in the lab, consisting of tubes and pumps that simulated blood vessels and a heart. Using this, they were able to show that the technique worked with up to four devices transmitting molecular signals at once, performing better than existing techniques.
Of course, success in lab tests doesn’t necessarily translate to real-life human use, and the team acknowledges that there are far more factors at play in live patients. However, they do say that this is a promising first step towards that eventual goal. Other scientists have found success transmitting data through ion exchange in human tissue.
The research was presented at the ACM SIGCOMM 2023 conference in September.
Source: EPFL