An innovative new method of drawing direct electrochemical energy from seawater means submersible robots, vehicles and detectors could go deeper and longer into the unknown.
The extreme conditions of the ocean depths present huge challenges for underwater exploratory, repair and monitoring devices, and being tethered via a powered-lifeline to a ship on the surface is not only limiting, it's often impossible. This means submersible craft and instruments really need their own onboard power supplies. Of course, this finite resource limits the range and the time submersible technology can work below the waves.
One way to deal with these limitations is to create supplemental electricity from the seawater itself. This technology exists – in varying stages of development – but researchers at East China Normal University (ECNU) have come at the problem from a slightly different angle.
Reporting in the journal Angewandte Chemie, Dr. Ming Hu from the School of Physics and Materials Science at ECNU and his colleagues were inspired by the ability of some marine organisms to switch cell respiration between aerobic and anaerobic, using different materials as electron acceptors.
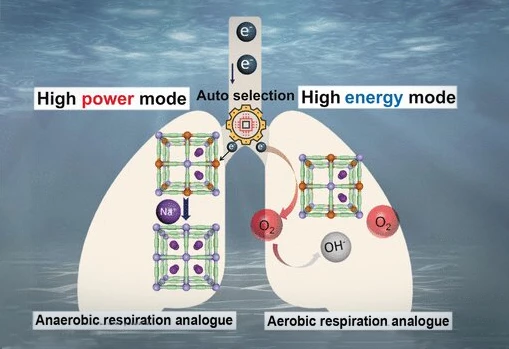
Employing these principals, the researchers designed a new kind of power generator, one which can efficiently and affordably meet the varying power demands of submersible technology. Their system not only provides reliable, long-term power, but it also flip-flops – autonomously – to suit either low-power tasks (such as lights, temperature-monitors and cruising) or brief, high-power tasks (such as acceleration or the operation of mechanical grippers).
This switchable power-generation is made possible using a cathode made of Prussian Blue. Yes, Prussian Blue pigment, like you'd see in blueprints. Prussian Blue is cheap and plentiful, which makes batteries that use it for their electrodes economically attractive. But the pigment has other benefits besides price-point. Its unique makeup allows it to store energy much more rapidly – and reversibly – than other compounds commonly used for battery electrodes. This makes it incredibly resilient, especially when it comes to charge/discharge cycles.
Prussian Blue has a structure with cyanide ions as "struts" and iron ions as "nodes" which readily accept and release electrons, and when combined with a metal anode, can be used to generate electricity from surrounding seawater.
The ability to autonomously switch modes to accommodate different power requirements is what makes this generator – or as the researchers call it "an auto-switchable dual-mode seawater energy extraction system enabled by metal–organic frameworks" – so interesting.
When power-demand is low, the system relies on dissolved oxygen. Since dissolved oxygen is plentiful in seawater (albeit at low concentrations), low-current power is – theoretically – unlimited. When a submersible requires a rapid increase in electrical current for more power-intensive tasks, the low concentration of oxygen is unable to rise to the challenge, but this is when the Prussian Blue cathode does its little flip-flop trick.
In response to the power-demand, abundant positively charged sodium ions – and therefore abundant electrons – are rapidly absorbed. When the demand abates, electrons are once again transferred to oxygen, the sodium ions depart and the generator is back in low-draw, long-term power mode.
The test-system – which the team states is corrosive-resistant and able to tolerate numerous mode-switches – successfully ran continuously in high-energy (i.e. low power) mode for four days straight with no apparent power-loss.
A paper on the development has been published in the journal Angewandte Chemie.
Source: East China Normal University