It’s an unfortunate irony that while many regions struggle to find enough water, there’s trillions of liters of the stuff floating around in the air everywhere. A new water harvester design from MIT can pull enough fresh water out of the air to meet the daily needs of several people.
Water harvesters are usually made up of adsorbent materials, meaning they collect water on their surfaces. To maximize the surface area exposed to the air, this new device is made up of a series of vertical fins spaced 2 mm (0.08 in) apart. These fins are made up of copper sheets, sandwiched in copper foams and then coated with a specialized zeolite material which is often used for water adsorption.
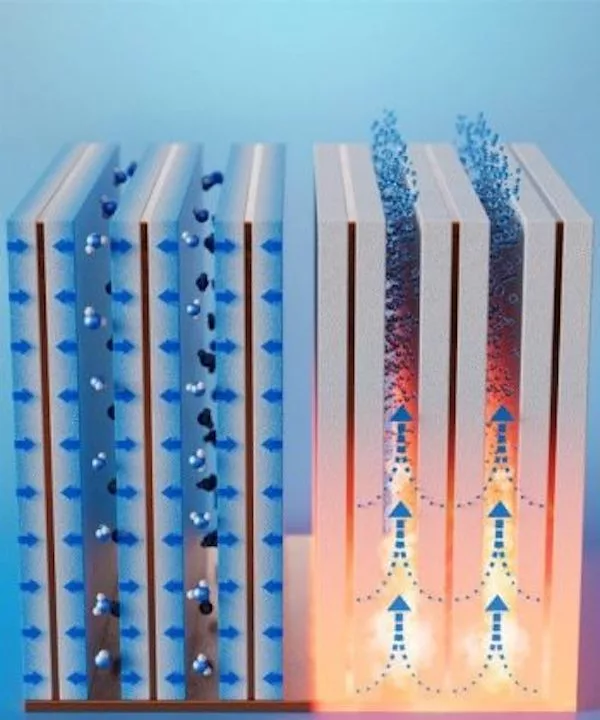
After an hour the fins are saturated with water, so the copper sheets are heated up to release it. If this cycle is performed 24 times per day, in air with 30% humidity (classed as arid), the team estimates the harvester can produce up to 1.3 L (0.3 gal) of drinkable water per day per liter of the adsorbent coating used. Scaled up, that’s 5.8 L (1.5 gal) per kilogram (2.2 lb) of material used per day, which is enough to satisfy several people’s daily water needs.
While there’s no shortage of other water harvesters in the works, this one has a few advantages. For one, it collects more water than most – some can only fetch 100 ml (1.5 oz) of water per kg of material. A Johns Hopkins design sounds particularly impressive, harvesting 8.66 L (2.3 gal) per day per kg of material, but these tests were conducted at 70% humidity. The new design can also work consistently throughout the day and night, where others collect their water overnight and release it in the morning.
The potential downside is that this system requires energy to release the water – the base of the device needs to reach 184 °C (363 °F) to wring it out. But the team says the device can tap into waste energy or heat from other systems, like buildings or vehicles.
The research was published in the journal ACS Energy Letters.
Source: American Chemical Society




