Not only have researchers identified how a common cellular protein affects aging, but they’ve tweaked the genes that produce it in fruit flies, extending healthy lifespan by 25% to 30%. The discovery opens the door to healthier aging in humans.
The cytoskeleton provides most cells with their shape, structure, and internal organization. In turn, the cytoskeleton relies on a type of actin protein, called filamentous or F-actin. It forms networks of thin, flexible filaments that affect the shape, stiffness, and movement of cells. Studies have found that aging alters actin expression, disrupting the cytoskeleton’s functions, which can lead to age-related diseases, including cancer and neurodegenerative diseases.
A new study by UCLA researchers has investigated the role of actin in brain aging and found that when F-actin accumulates in the brain, it hinders cellular cleanup and leads to the buildup of waste that decreases neuronal functioning and contributes to cognitive decline. However, they also found that tweaking some genes in fruit flies prevented the build-up of F-actin and extended the flies’ healthy lifespan by around 30%.
“Flies get more forgetful as they age, and their ability to learn and remember declines in middle age, just like it does in people,” said David Walker, the study’s corresponding author and a professor from UCLA’s Department of Integrative Biology and Physiology. “If we prevent accumulation of F-actin, it helps the flies learn and remember when older – which tells us the buildup is not benign.”
Autophagy (from the Ancient Greek for ‘self-eating’) is the body’s cellular recycling system. This vital process breaks down and cleans out old, damaged, or abnormal proteins and other cellular substances. There’s growing evidence that autophagic activity declines with age, including in the brain.
The researchers experimented on a Drosophila – fruit fly – model, examining the F-actin in the brains of naturally aging animals. They compared the brains of young, middle-aged, and late-age flies and observed a significant increase in total F-actin levels in brains as they aged.
To determine if the F-actin levels they observed reflected age or occurred universally over time, the researchers then examined flies on a dietary and/or protein restriction, an approach that’s been shown to slow aging and promote longevity. They found that flies fed a low-protein diet had a significantly longer lifespan than those fed a high-protein diet. Further, they saw F-actin in the brains of flies on a rich diet at young middle age that wasn’t seen in the brains of flies on a dietary restriction.
Rapamycin, a small molecule that has been shown to prolong the lifespan, was given to the flies next. Feeding flies rapamycin significantly extended their lifespan compared to those fed a control. In addition, aged flies fed rapamycin had significantly less F-actin in the brain than age-matched controls. All of the findings, taken together, suggested that age-associated F-actin reflected healthy aging in fruit flies and could be counteracted by strategies to improve longevity.
“But that’s correlation, not a direct demonstration that F-actin is detrimental to aging of the brain,” Walker said. “To get at causality, we turned to genetics.”
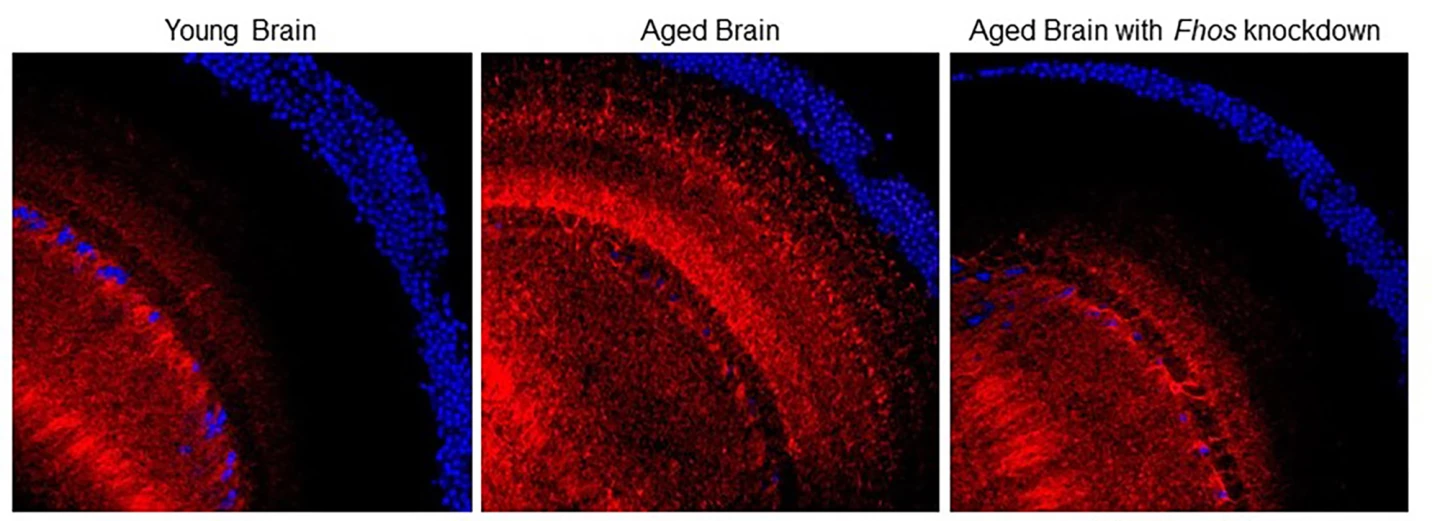
Because the fruit fly genome has been completely mapped, the researchers could target aging fly genes known to play a role in the accumulation of actin filaments. They found that knocking down the Formin homology 2 domain containing ortholog (Fhos) gene in fruit fly neurons prevented F-actin accumulation in the brain.
“When we reduced Fhos expression in aging neurons, it prevented the accumulation of F-actin in the brain,” said Edward (Ted) Schmid, who worked in Walker’s UCLA lab and is the study’s lead author. “This really allowed us to expand our studies because now, we had a direct way to target F-actin accumulation in the brain and study how it affects the aging process.”
Although the genetic ‘tweak’ targeted only neurons, the researchers saw that it improved the flies’ overall health. They lived 25% to 30% longer and showed signs of improved brain function and markers of improved health in other organs. Preventing the accumulation of F-actin protected cognitive function, suggesting that the buildup drives age-related cognitive decline.

A closer examination revealed that the F-actin had messed up the cell’s recycling system. The researchers found that preventing F-actin accumulation, caused more autophagy in the brains of aged fruit flies. If they removed F-actin and disabled autophagy, aging wasn’t slowed. It appeared that the primary mechanism by which F-actin was driving brain aging was by impairing autophagy. The researchers also showed that disrupting F-acting in aged brains restored brain autophagy to levels seen in youth and reversed certain cellular markers of brain aging.
Of course, these findings need to be translated into humans, which may prove to be more of a challenge. But challenges are what researchers are here for, right?
“Most of us in the aging field are focused on moving beyond lifespan into what we call the healthspan,” said Walker. “We want to help people enjoy good health and a high quality of life while extending the lifespan. Our study improved cognitive and gut function, activity level, and overall healthspan of fruit flies – and offers hope for what we might be able to achieve in humans.”
The study was published in the journal Nature Communications.
Source: UCLA