Scientists that won an infamous 2024 IgNobel Prize for "discovering that many mammals are capable of breathing through their anus" may indeed have the last laugh. They've now completed a successful human trial testing the safety and tolerability of enteral ventilation, a technique that gets oxygen into the body via an unconventional route.
Japanese and US researchers, led by the Cincinnati Children's Hospital, have completed the first-ever human trial testing the viability of enteral ventilation, where patients with severe respiratory failure could potentially have oxygen delivered through the intestine, allowing the lungs to recover and to prevent further injury. The procedure's safety and tolerability was examined on 27 healthy male adults in Japan, who had oxygen-rich fluid pumped into their anus.
“This is the first human data, and the results are limited solely to demonstrating the safety of the procedure and not its effectiveness," said researcher Takanori Takebe, MD, PhD, from the Cincinnati Children’s and the University of Osaka. "But now that we have established tolerance, the next step will be to evaluate how effective the process is for delivering oxygen to the bloodstream."
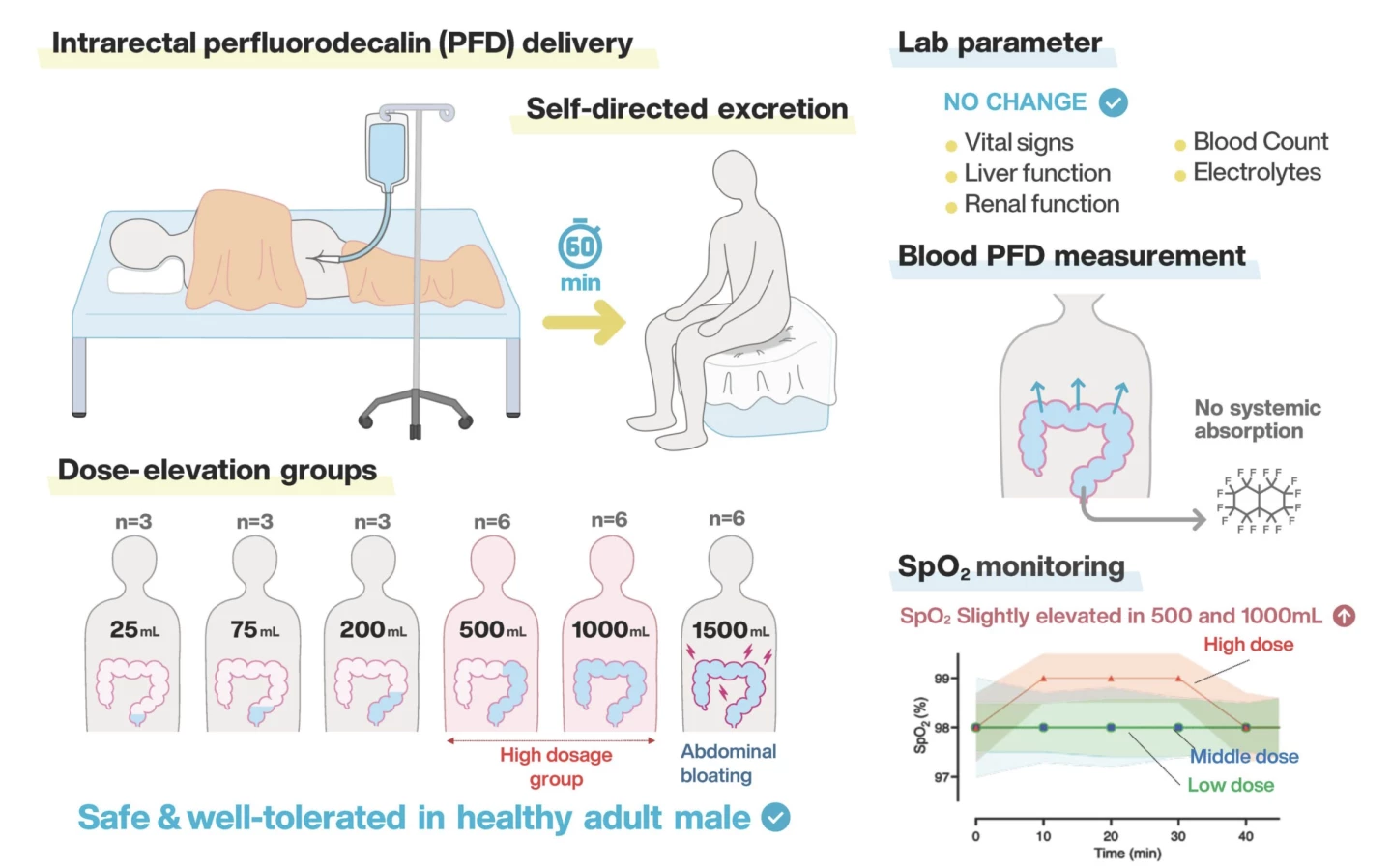
The brave volunteers, aged 20-45 years, received a single intrarectal dose of non-oxygenated perfluorodecalin liquid (up to 1,500 ml), which they were required to retain for 60 minutes. Safety and tolerability were assessed by monitoring of adverse events, vital signs, clinical laboratory tests and systemic perfluorodecalin exposure. And a model using large-animal data was used to predict potential oxygen transfer. Perfluorodecalin was used due to its excellent oxygen-carrying abilities.
Twenty of the volunteers held the liquid for 60 minutes, and at the largest volume of 1,500 ml, there were only mild side effects of abdominal bloating and discomfort. Meanwhile, all clinical laboratory measures, including liver and renal function markers, remained within normal range.
"This first-in-human study demonstrates that intrarectal administration of non-oxygenated perfluorodecalin is safe, feasible, and well tolerated," noted the researchers. "These findings establish a critical safety foundation and support the continued development of enteral ventilation with fully oxygenated perfluorodecalin as an adjunctive strategy to support respiratory failure patients."
This research first came about after scientists had studied a type of bottom-feeding fish that swallows air from the surface of the water and absorbs oxygen through its gut, supplementing gill function in poor quality conditions. If humans were able to safely absorb super-oxygenated liquid through their colon, into their bloodstream, it has the potential to provide life-saving emergency treatment to people with blocked airways caused by injury or inflammation, or when lung function has been severely compromised.
The scientists now plan to repeat the trial to measure how much of the liquid is needed, and for how long, to improve blood-oxygen levels.
The research was published in the Cell Press journal Med.
Source: Cincinnati Children’s Hospital






