Scientists found that nearly every cancer harbors its own distinct community of microbes – tiny passengers that can influence how tumors start, spread, and respond to treatment, paving the way for a new era of precision medicine.
We now know from abundant research that the gut microbiome – the ecosystem of trillions of bacteria, viruses, fungi, and archaea that live in our gastrointestinal system – is intrinsically linked to health.
A new study has summarized what’s currently known about the communities of microorganisms that live inside cancer tissue – the tumor microbiome – in different types of cancer. It looks at their influence on how cancers start, grow, spread, and respond to treatment – knowledge that could transform the way cancers are diagnosed, treated, and monitored in the era of precision medicine.
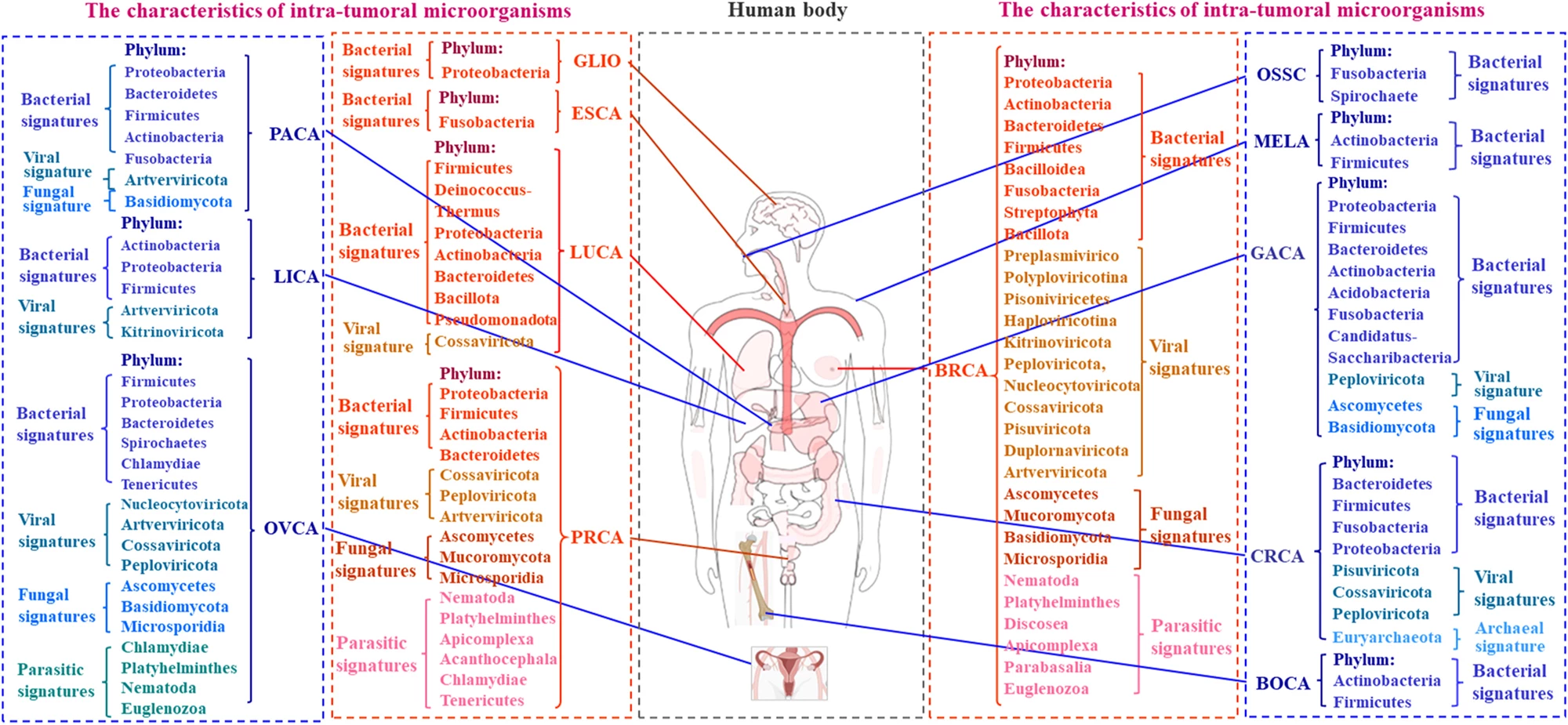
The researchers looked at findings by cancer type: breast, lung, prostate, pancreatic, stomach (gastric), bowel (colorectal), ovarian, melanoma, liver, esophageal, brain and bone cancers. For each type, they determined the distinct microbial “signature.” For example, lung tissue, once thought to be sterile, hosts microbes like Proteobacteria and Firmicutes, and cancerous lungs tend to have lower bacterial diversity, with Streptococcus and Neisseria often found in higher levels. Prostate cancer contains a variety of microbes, especially Proteobacteria and Actinobacteria, and viruses and fungi are also found. Ovarian tumors contain Firmicutes, Proteobacteria, Chlamydia, Mycoplasma, and various fungi; human papillomavirus (HPV) and cytomegalovirus (CMV) infections are frequent.
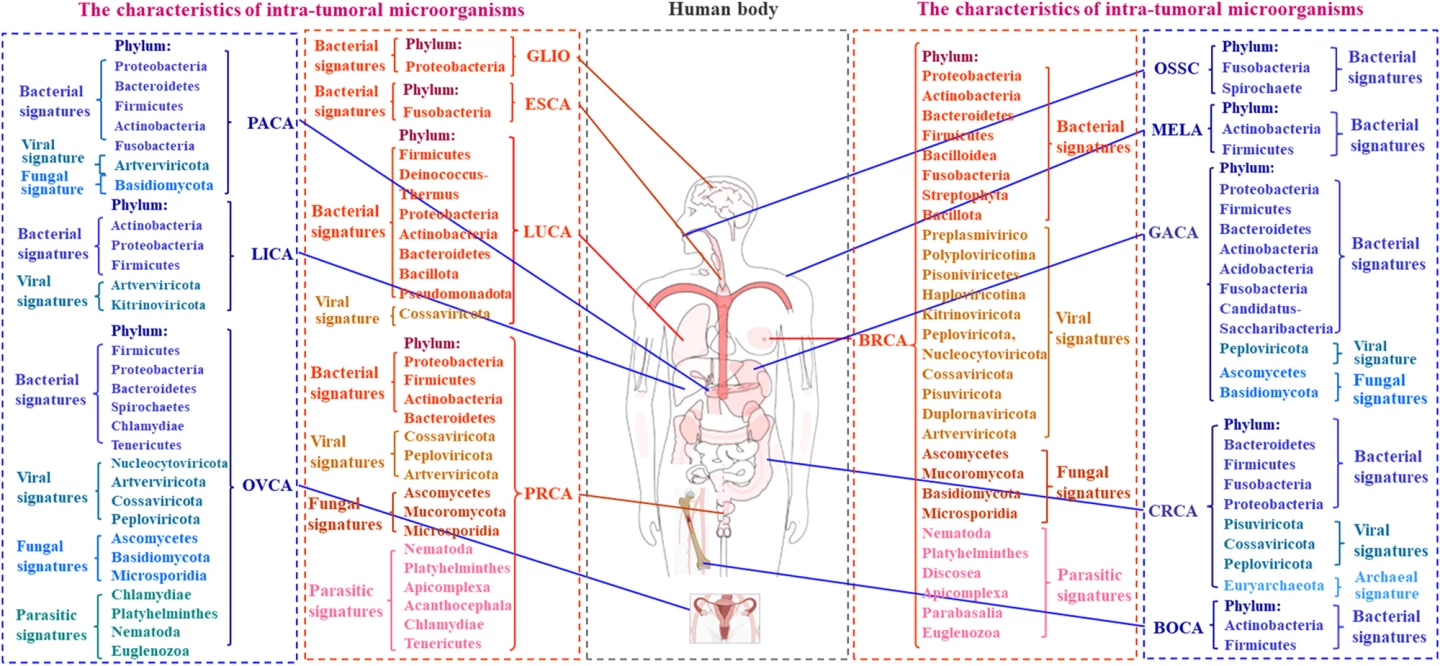
They also determined the effect of each microbial community. Specific bacteria such as Thermus and Legionella were linked with advanced lung cancers and cancer spread (metastasis), for example. In pancreatic cancer, microbial diversity correlated with survival, suggesting its potential as a prognostic marker. Likewise, colorectal cancer, where microbiota composition correlated with tumor stage and survival.
The researchers proposed several mechanisms by which microbes influence cancer. Some insert their DNA into host genomes – for example, HPV in ovarian cancer – or produce toxins that damage DNA (E. coli), which triggers mutations. That’s one theory. Another is that microbial metabolites can activate cancer-promoting signaling pathways that encourage tumor growth and spread. A third is that microbes alter local immune balance, suppressing anti-tumor immunity or promoting inflammation. For example, Fusobacterium nucleatum reduces T-cell activity, which is seen in colorectal and esophageal cancers. Some species produce metabolites that may inhibit tumor invasion.
Nearly all cancers harbor their own distinct microbial ecosystems. These microbes can promote or suppress tumor growth, alter immune response, and affect treatment outcomes. Understanding and targeting the tumor microbiome could have a few flow-on effects. It could lead to earlier, more accurate diagnoses, enable personalized treatments based on microbial profiles, and could help overcome drug resistance and improve survival rates.
However, more research is needed to determine whether these microbes cause cancers or simply adapt to the tumor environment.
The study was published in the journal Genes & Diseases.