Scientists have engineered Salmonella bacteria to self-destruct inside tumors, releasing signals that spark powerful immune hubs and shrink colon cancer in mice, opening the door to “living medicines” against deadly cancers.
Colorectal cancer is one of the deadliest cancers worldwide, and current immunotherapies often don’t work well to treat it. In an effort to reduce its high mortality rate, scientists are continually seeking new and more effective treatments for colon cancer.
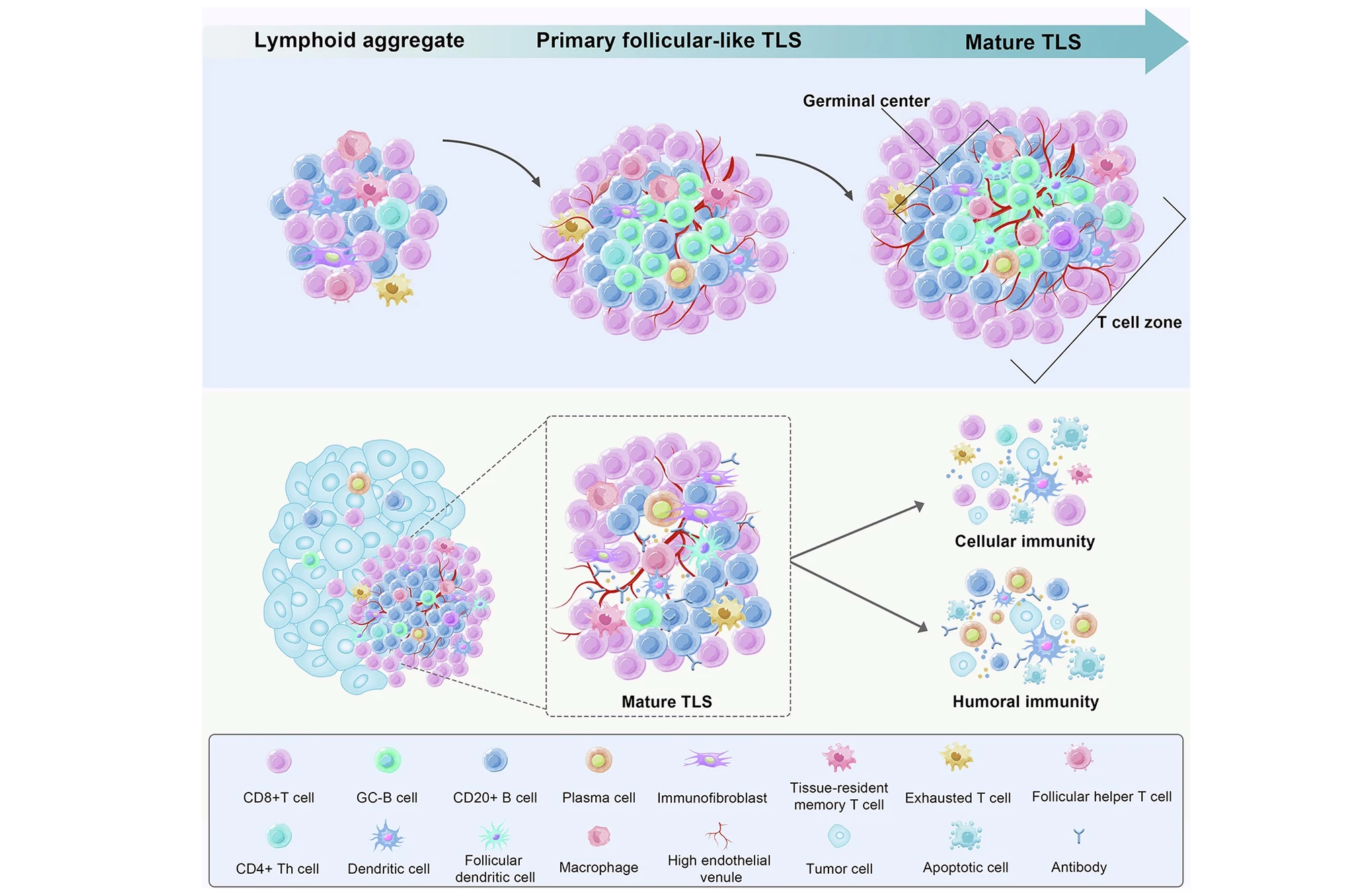
In a new study by the National University of Singapore’s Yong Loo Lin School of Medicine (NUS Medicine) and Central South University, China, researchers have explored a promising avenue in colorectal cancer treatment, boosting special immune cell clusters called mature tertiary lymphoid structures (mTLSs), that form near tumors and are linked to better survival.
“This work provides compelling evidence that mTLSs can be therapeutically induced using synthetic biotics,” said co-corresponding author Xiaoyuan (Shawn) Chen, PhD, Professor in Medicine and Technology and Director of the Nanomedicine Translational Research Program (TRP) at NUS Medicine. “Our engineered strain stimulates a key immune signaling pathway, LIGHT-HVEM, to activate group 3 innate lymphoid cells and kickstart T cell-mediated antitumor responses.”
That probably reads like scientific gobbledygook to many, so let’s break it down into more understandable terms, including how the Salmonella bacterium factors into the equation.
The researchers started with a weakened strain of Salmonella typhimurium, which has already been shown to be safe in earlier human trials for other cancers. This strain naturally homes in on tumors. They engineered the bacteria by adding a synchronized lysis circuit (SLC), so they would self-destruct in unison once they reached high density inside tumors. Upon this lysis, the bacteria release a protein called LIGHT, which binds to a receptor called HVEM on immune cells, thereby driving strong immune activation.
The therapy was tested in two types of colorectal-cancer-prone mice: a genetic model (mice that naturally develop intestinal tumors) and a chemical model (where cancer was induced). The researchers measured shifts in innate immune cells, particularly ILC3s, T cells, and the presence of mTLSs. ILC3s are group 3 innate lymphoid cells, an immune cell that is crucial for maintaining health in barrier tissues like the gut and lungs.
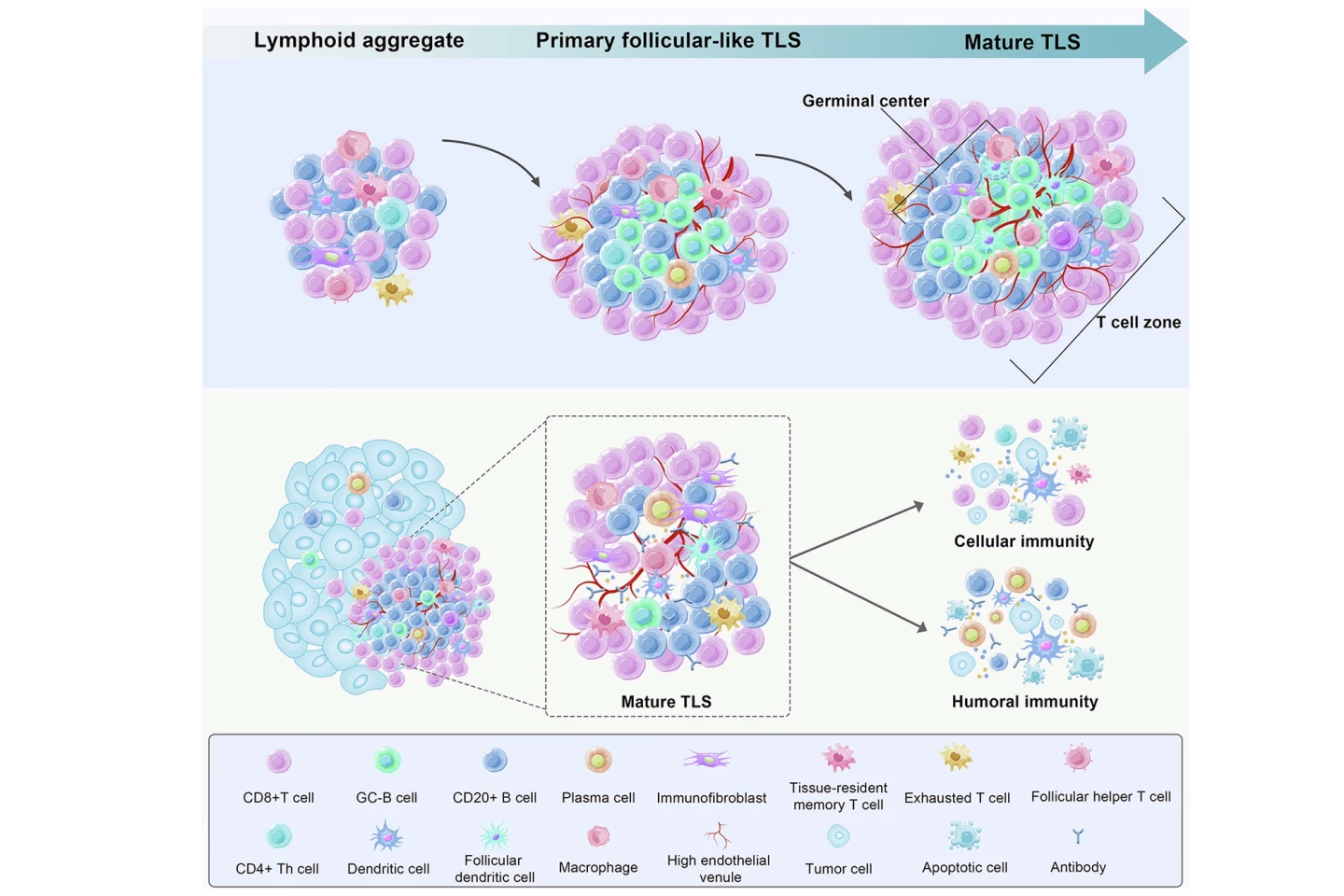
The researchers knew that normal colorectal cancer development shifted these protective ILC3 cells into less helpful ILC1s. However, they found that treatment with engineered Salmonella reversed this effect: ILC3s increased, while ILC1s decreased. And whereas plain S. typhimurium boosted tertiary lymphoid structures (TLSs) but didn’t mature them enough for strong tumor-fighting, the LIGHT-releasing therapy not only increased TLSs but “upgraded” them to mTLSs with organized B-cell and T-cell zones, a sign of effective immune niches.
The therapy also produced stronger antitumor immunity. CD8+ T cells (killer T cells) were more active, producing interferon-gamma (IFN-γ), which stimulates immune responses and prevents tissue damage, and granzyme B, which directly induces programmed cell death (apoptosis) in target cells to eliminate cancer or infection. Tumor growth was significantly reduced, survival was improved, and some mice achieved complete tumor control. The treatment benefit relied on LIGHT-HVEM signaling and ILC3s. In mice lacking HVEM or ILC3s, the therapy failed to mature TLSs or shrink cancer tumors.
The study has some limitations, notably that the effects were seen in mice; human immune systems and gut bacteria may respond differently. Engineered bacteria release many factors upon lysis, so it’s hard to pinpoint which effects are LIGHT-specific. The exact subtypes of ILCs and downstream pathways are still unclear. Finally, any live bacterial therapy carries the risk of unintended infection, inflammation, or unpredictable interactions with the patient’s microbiota.
Nonetheless, if the findings are translatable to humans, this approach could provide a new therapeutic angle, using a living engineered bacterium that colonizes tumors and delivers immune-stimulating molecules on-site. The therapy could complement checkpoint inhibitors or cancer vaccines by making colorectal cancer tumors more “immune-visible.” The same strategy might be adapted for other hard-to-treat solid tumors by changing the cargo the bacteria deliver.
“This approach could pave the way for programmable ‘living medicines’ that reshape the tumor environment from within,” said Pengfei Rong, MD, PhD, the study’s other corresponding author, from the Third Xianguya Hospital, Central South University.
The researchers will continue testing their therapy, with the goal of advancing it toward human clinical trials.
The study was published in the journal Science Translational Medicine.
Source: NUS Medicine