Tiny "hidden" proteins lurking in DNA once dismissed as junk may hold the key to the next generation of obesity drugs, according to a new study that has uncovered dozens of new fat-regulating molecules using cutting-edge gene-editing technology.
GLP-1 drugs like Ozempic and Wegovy have taken the weight loss world by storm. And they’ve started a revolution to discover other drugs that are effective at treating obesity, the worldwide prevalence of which has more than doubled since 1990, and that is closely associated with diseases such as diabetes and heart disease.
In their search for a new obesity treatment strategy, researchers from the Salk Institute for Biological Studies in California have used CRISPR/Cas9 gene editing technology to closely examine an understudied class of molecules once thought to be junk: microproteins. They’ve presented their findings in a recently published study.
“CRISPR screening is extremely effective at finding important factors in obesity and metabolism that could become therapeutic targets,” said the study’s co-corresponding author, Professor Alan Saghatelian, head of the Salk Institute’s Clayton Foundation Peptide Biology Laboratories, otherwise known as the Saghatelian Lab. “These new screening technologies are allowing us to reveal a whole new level of biological regulation driven by microproteins. The more we screen, the more disease-associated microproteins we find, and the more potential targets we have for future drug development.”
Microproteins are tiny proteins, often made from “small open reading frames” (smORFs) – previously overlooked sections of our DNA once thought to be junk – that can have surprisingly big effects on how the body works. They’re typically less than 100 amino acids long, but they can act like switches or fine-tuners for important biological processes such as controlling how cells grow, how they use energy, how the immune system responds, or how tissues develop. In health, microproteins help keep body systems balanced, but when they’re missing, overactive, or faulty, they can contribute to diseases ranging from metabolic disorders and cancer to heart disease and immune problems. GLP-1 is a peptide that’s small enough to be considered a microprotein, according to the researchers.
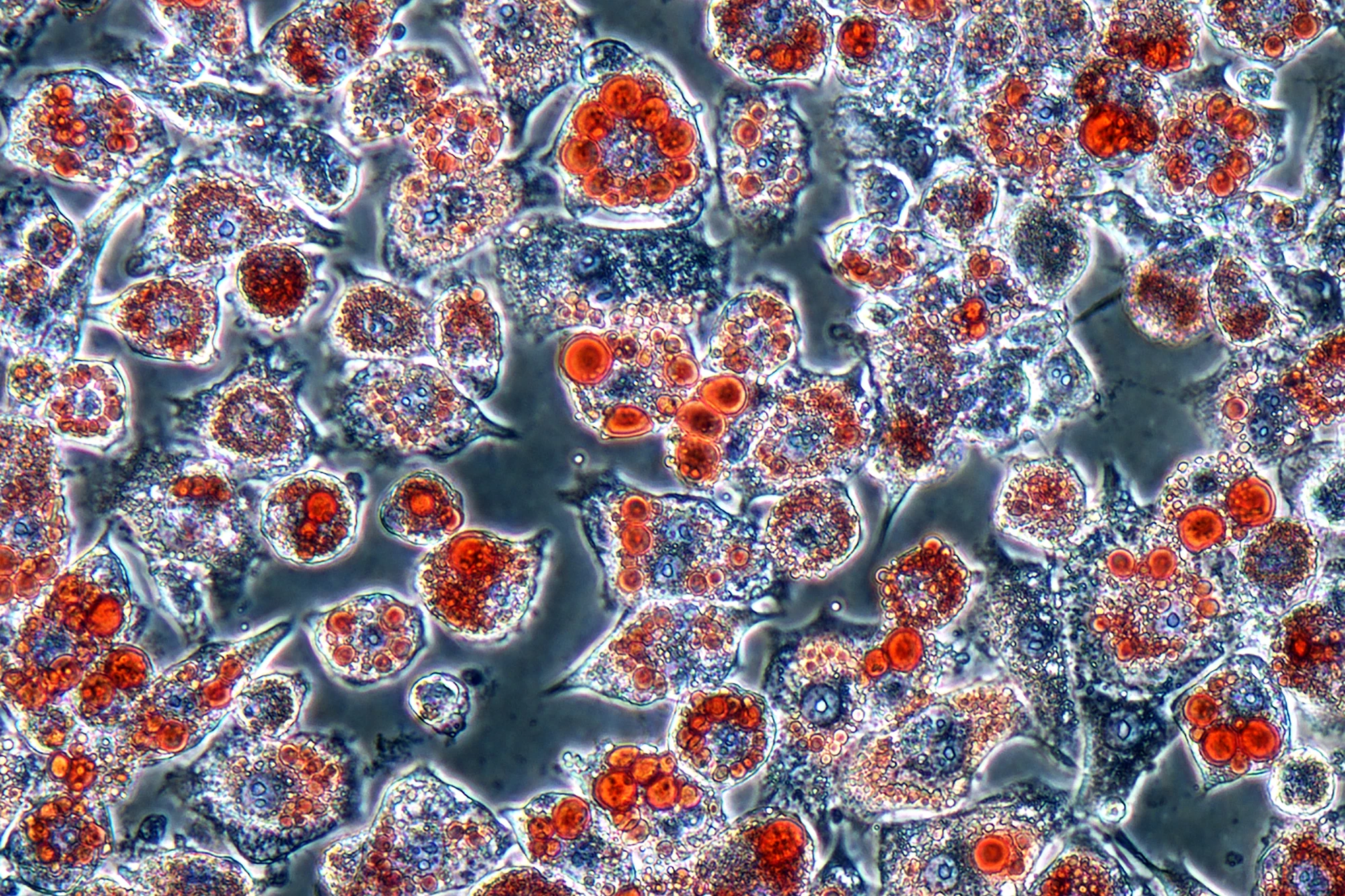
In the present study, the researchers used CRISPR/Cas9 gene editing to screen thousands of genes in mouse fat cells, or adipocytes, to see which ones might produce microproteins that influence fat cell growth (proliferation) and fat storage. From this massive screen, they identified dozens of potential microproteins. From that shortlist of 38 candidates, one microprotein, called Adipocyte-smORF-1183, actually affected how fat cells develop and store fat.
“We wanted to know if there was anything we had been missing in all these years of research into the body’s metabolic processes,” said lead and co-corresponding author Victor Pai, a postdoctoral researcher in Saghatelian’s lab. “And CRISPR allows us to pick out interesting and functional genes that specifically impact lipid accumulation and fat cell development. We’re not the first to screen for microproteins with CRISPR, but we’re the first to look for microproteins involved in fat cell proliferation. This is a huge step for metabolism and obesity research.”
While Adipocyte-smORF-1183 can still only be considered a “potential” obesity treatment at this stage, its discovery points toward a new class of drug targets that could work differently from current treatments, be more precise, reduce unwanted side effects, and open the door to “microprotein-based” therapies for obesity and metabolic disorders. Nonetheless, the takeaway message from this research is that we may be standing on the edge of discovering new obesity drugs from the parts of our DNA we once thought were useless.
Next steps will include repeating the process using human fat cells to identify microproteins that could be directly relevant for human treatments, and to continue working through the shortlist of 38 microproteins identified by the researchers.
“That’s the goal of research, right?” Saghatelian said. “You keep going. It’s a constant process of improvement as we establish better technology and better workflows to enhance discovery and, eventually, therapeutic outcomes down the line.”
The study was published in the journal Proceedings of the National Academy of Sciences (PNAS).
Source: Salk Institute