A fundamental breakthrough in chemistry promises to unlock ammonia as a clean fuel, and it could help decarbonize the entire chemical industry in the process. Rice University researchers have created a small, LED-powered device that converts ammonia to hydrogen on the fly. It uses a light-driven catalyst that's as efficient as expensive thermal catalysts that need thousand-degree temperatures to operate, and it's made from cheap, abundant copper and iron. And it's only the beginning of a technology that could radically reduce costs and energy use in industrial chemistry.
Hydrogen is a very promising clean fuel that can be burned, or converted directly into electricity through a fuel cell. It's both expensive and difficult to handle, though, since it's a super-lightweight gas that needs to be compressed to 700 atmospheres, or else cryogenically cooled within sight of absolute zero to reach its liquid state.
Ammonia is famously a better hydrogen carrier than hydrogen gas itself; each of its nitrogen atoms binds three hydrogen atoms, and while it's caustic and extremely hazardous in high concentrations, it's a stable liquid at atmospheric temperatures and pressures, and its widespread use in many industries means people have plenty of experience handling it safely under a wide range of conditions.
Ammonia might carry hydrogen exceptionally well, but if you want to use that hydrogen, you need to "crack" it to get the hydrogen out and release the harmless nitrogen back into the atmosphere. This has been difficult for two main reasons: firstly, the reaction is endothermic, so most ammonia cracking is done in large facilities operating at temperatures of at least 650-1,000 °C (1,200-1,800 °F). Secondly, the thermal catalysts required for the cracking operations are typically platinum-group metals like ruthenium – relatively rare and expensive.
With the green hydrogen movement gathering steam as a key pillar of the transition to clean energy, you can see why the Rice University team is excited to have discovered a compact and efficient way to catalyze this cracking reaction at room temperature, using nothing but copper and iron.
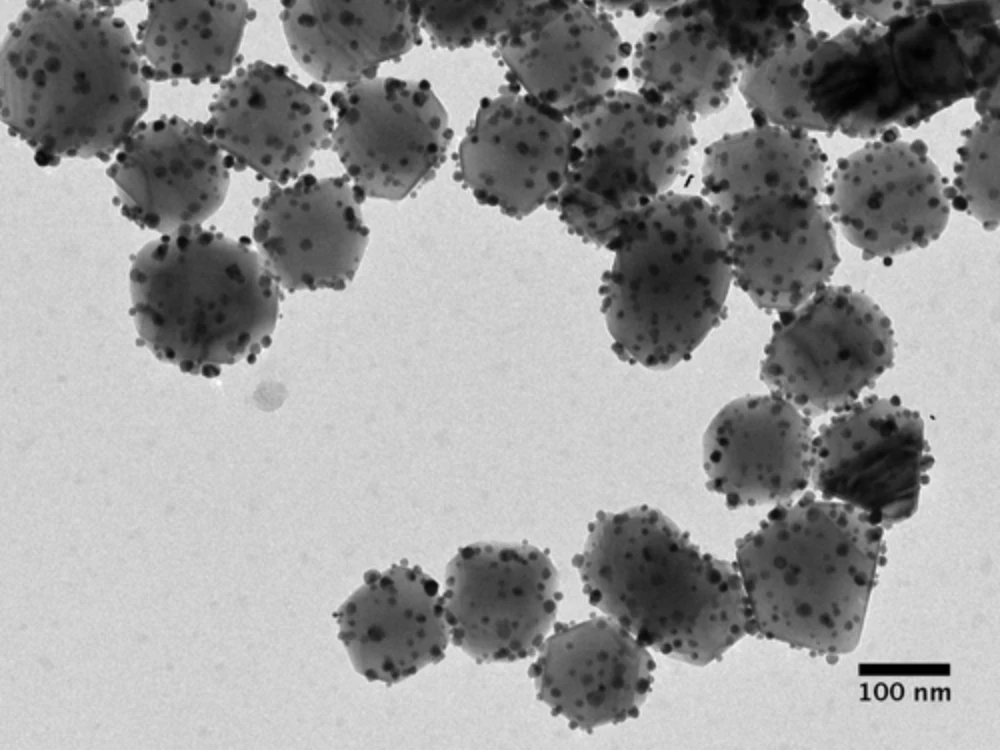
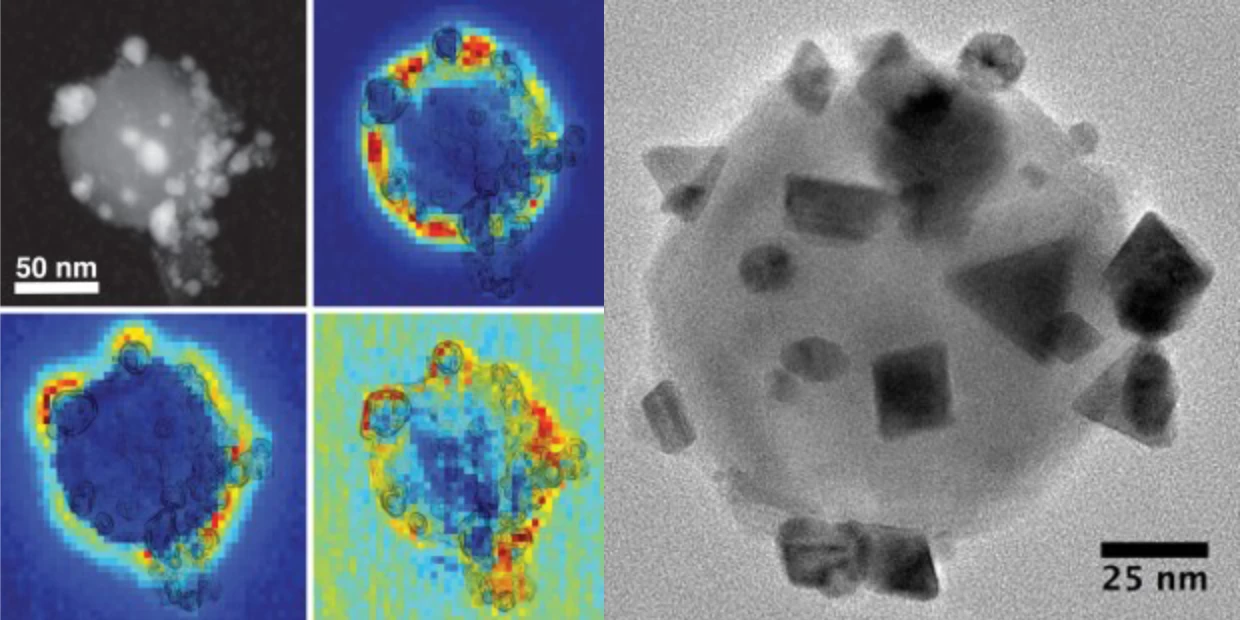
It comes down to photocatalytics; this team has been working for more than 30 years to develop its "antenna-reactor" plasmonic photocatalysts. These are nanoparticles of a catalyst, dotted with little clumps of an "antenna" material designed to increase the catalyst's ability to absorb light. Properly tuned, these antenna-reactor particles take in energy from ambient light – be it sunlight, or light from low-energy LEDs – and kick out short-lived "hot electrons" with enough energy to start an efficient chemical reaction even at ambient temperatures.
Antenna-reactor photocatalysts can be designed for all sorts of reactions. This is the same team, and essentially the same underlying idea, behind the light-powered hydrogen sulfide-to-hydrogen catalyst we wrote about a few weeks ago, for example. That one used silicon dioxide as the "reactor" catalyst, with tiny particles of gold as the "antenna" drawing energy in from light.
This ammonia-splitting photocatalyst uses iron as its reactor, and copper as its light-collecting antenna – both cheap and abundant metals, as opposed to the typical copper-ruthenium thermal catalysts used today. In lab testing, according to Rice alumnus and study co-author Hossein Robatjazi, "under illumination, the copper-iron showed efficiencies and reactivities that were similar to and comparable with those of copper-ruthenium."
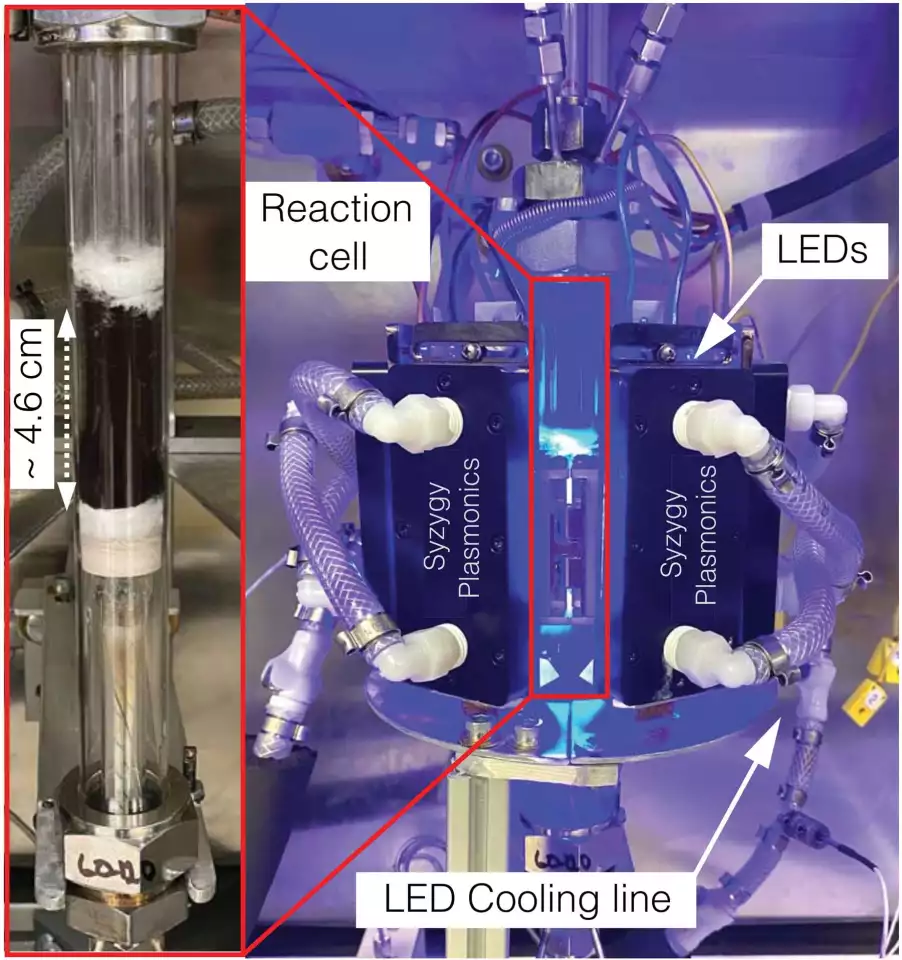
The initial tests were conducted using light supplied by lasers, in a tiny experimental setup. But study co-author Naomi Halas is also a co-founder of Syzygy Plasmonics, a well-funded company set up to commercialize the Rice team's work, and Syzygy was able to license this particular catalyst and build a test rig some 500 times larger, using efficient LED lighting instead of lasers. The catalyst remained just as efficient.
“This is the first report in the scientific literature to show that photocatalysis with LEDs can produce gram-scale quantities of hydrogen gas from ammonia,” Halas said. “This opens the door to entirely replace precious metals in plasmonic photocatalysis.”
So, going back to why we should care about all this, the copper-iron photocatalyst should make it much cheaper and easier to extract hydrogen from ammonia. But it'll also do it without needing heat, so there'll be energy and emissions saved as well.
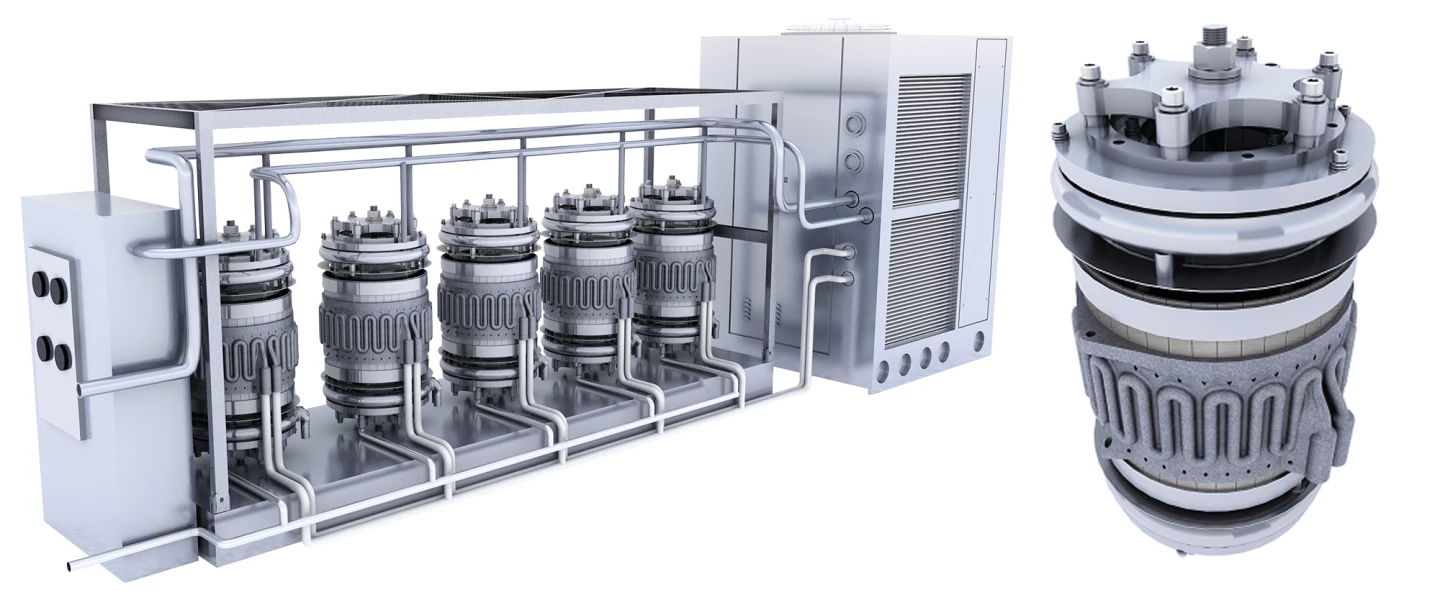
Perhaps most importantly, this looks like it'll lead to an ammonia cracking device that's small, reliable, lightweight, and cool rather than running at hundreds of degrees. Something that doesn't need a full-scale facility to operate. Syzygy says its initial Rigel Photocatlytic Reactor product is about the size of a small washing machine, and processes around a ton a day, depending on the specific reaction it's running. These can be stacked; you can run a bunch of them simultaneously if you need a greater output.
Perhaps you could stick a bank of these on an electric cargo ship, converting easily-stored ammonia into easily-used hydrogen right where it's needed. That in itself could be absolutely revolutionary, radically boosting the range of clean cargo and passenger shipping.
Perhaps the concept might prove small and light enough to be relevant in aviation, where the energy density of hydrogen stored in ammonia could open up range figures that are currently out of reach without jet fuel. Perhaps it might eventually be small enough to stick in an electric car you can fill up with ammonia at a gas station.
And that's just this particular photocatalyst; the Rice and Syzygy teams are certainly not stopping here. Indeed, the company's target is to put thermal catalysts out of a job wherever they can.
“Given their potential for significantly reducing chemical sector carbon emissions, plasmonic antenna-reactor photocatalysts are worthy of further study,” added another co-author, Emily Carter. “These results are a great motivator. They suggest it is likely that other combinations of abundant metals could be used as cost-effective catalysts for a wide range of chemical reactions.”
"Catalysis is the foundation of chemical industry," says yet another co-author and Syzygy co-founder Peter Nordlander, "and it's one of the most energy-consuming parts of all society."
"We show in this work," adds Halas, "that LED-based chemistry is actually doable, and it's doable at scale. It can contribute to industrial-scale chemistry, and industrially-important reactions."
Syzygy says it's already got this reaction up and running in field trials, and that it expects to have these photocatalytic ammonia-cracking reactors commercially available in 2023.
This is some very exciting technology, with huge potential across a range of industries and as a contributor to decarbonization. Learn more in the video below.
Source: Rice University








