Green hydrogen can't be viewed as environmentally friendly if it drinks huge amounts of fresh water, or results in the bulk output of toxic chlorine, according to RMIT researchers who say they've come up with a cheap technique that does neither.
Research into green hydrogen production is advancing at a rapid rate right now, as countries worldwide jockey to establish themselves in what's expected to become a huge global market for clean fuels. Australia's vast renewable energy potential and export-focused economy place it well to compete at high volumes internationally. But as a continent dominated by desert, it's also critically aware of water shortages and the dangers of shipping the lifeblood of its land overseas. Nine liters (2.4 gal) of fresh water per kilogram (2.2 lb) of hydrogen bodes poorly for bulk production.
Creating green hydrogen from seawater is harder than using fresh water; there's corrosion to think about, as well as lots of impurities and micro-organisms. You need coastal locations close to renewable energy – not a problem for a country as large and relatively empty as Australia, but definitely a factor elsewhere. At some scale you need to think about what you're putting back into the ocean after you've finished – whether you're creating dangerous levels of salinity or pumping high concentrations of toxic chlorine back into the marine environment.
But the benefits are pretty huge; not only is your water supply free when you use seawater, but if that hydrogen is burned or run through a fuel cell locally, fresh water is emitted that can filter through the water table and feed the parched land. Desalinated water is one heck of a bonus.
Hence, there are currently lots of teams working on electrolysis techniques that generate green hydrogen from seawater. Back in December we looked at an efficient Chinese device that uses vapor pressure differentials to spontaneously evaporate pure water out of seawater, and then electrolyzes that. A couple of weeks ago, we highlighted an international team that has found a surface treatment for standard electrolyzers that converts them to run just as well on seawater. And back in 2021, we looked at a very exciting method out of Saudi Arabia that captures not only hydrogen, but saleable quantities of chlorine and battery-grade lithium phosphate, which would address an additional global problem and look like pretty darn good business in the process.
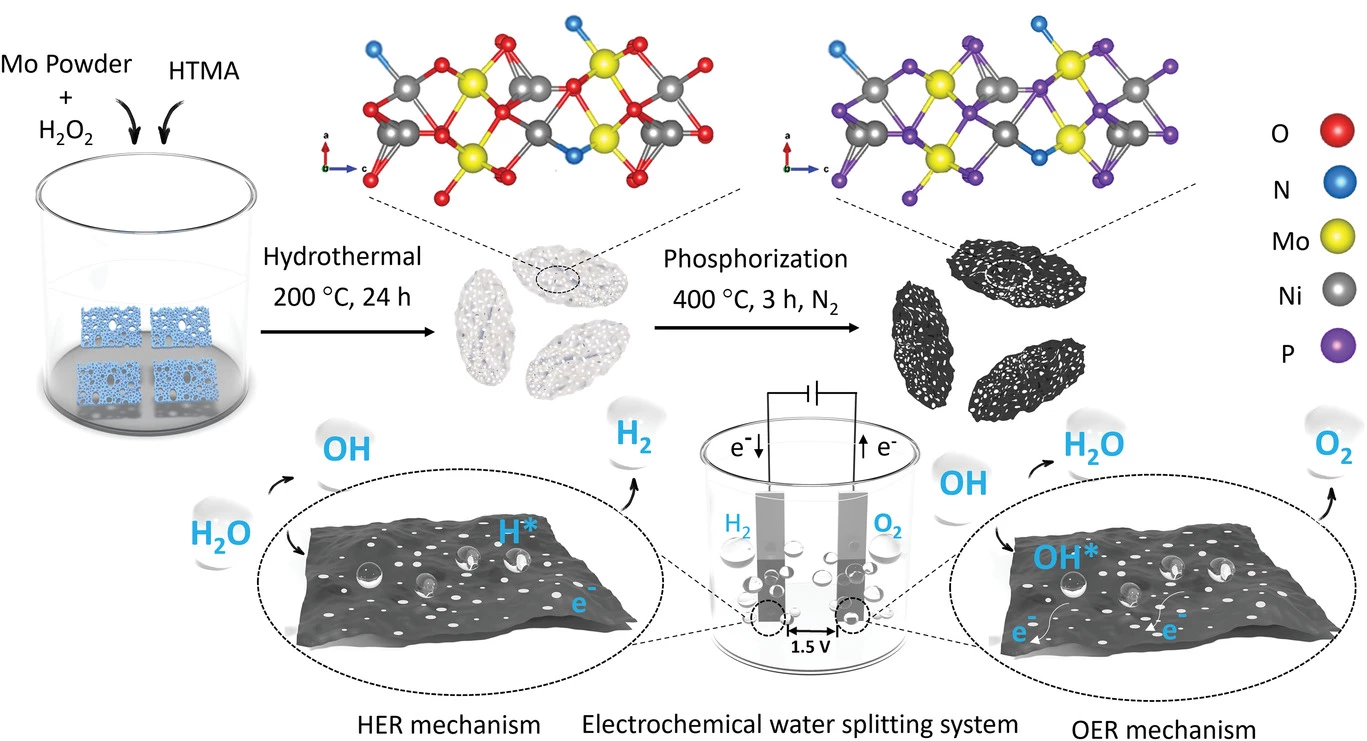
Today, scientists at Australia's RMIT announced another approach with great potential for highly efficient, low-cost green hydrogen generation straight from seawater, without generating chlorine.
"The biggest hurdle with using seawater is the chlorine, which can be produced as a by-product." said Dr Nasir Mahmood, lead researcher on a paper just published in the peer-reviewed Wiley journal Small. "If we were to meet the world’s hydrogen needs [using seawater] without solving this issue first, we’d produce 240 million tons per year of chlorine each year – which is three to four times what the world needs in chlorine. There’s no point replacing hydrogen made by fossil fuels with hydrogen production that could be damaging our environment in a different way. Our process not only omits carbon dioxide, but also has no chlorine production."
The RMIT device uses a novel catalyst made from sheets of nitrogen-doped nickel molybdenum phosphide (NiMo3P). Throughout each sheet layer, there are large pores – well, large at the nanoscale, anyway – designed to accelerate catalytic activity and mass transfer.
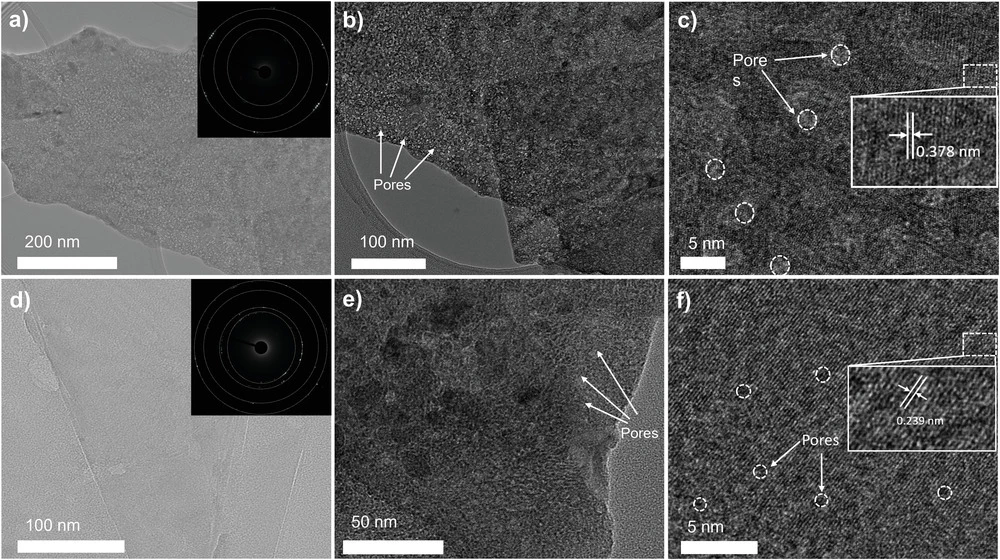
The nitrogen doping, says the team, performs a number of functions, including increasing conductivity, optimizing the electronic density and surface chemistry, and creating new active sites for water catalysis in the sheets. The electro-negative properties that arise when nitrogen bonds to the surface metals helps to stop unwanted ions and molecules from touching the surface of the catalyst, and the presence of phosphate, sulfate, nitrate and hydroxyl ions on the surface serve to block out chorine and prevent corrosion.
Experimentally, the team found this catalyst exhibited outstanding efficiency, and completely suppressed the generation of chlorine. "The N-NiMo3P sheets exhibit exceptional HER [hydrogen evolution reaction] overpotential values of 23 and 35 mV at 10 mA cm−2 in alkaline electrolytes and seawater, respectively," reads the study. "Besides, for full water splitting, it only requires 1.52 and 1.55 V to achieve 10 mA cm−2 in alkaline electrolyte and seawater, respectively. These exceptional results demonstrate that low-cost hydrogen can be generated from seawater by regulating the structure and composition of 2D materials."
“These new catalysts take very little energy to run and could be used at room temperature,” Mahmood clarifies in a press release. They should also be relatively cheap and easy to produce at the huge scale the green hydrogen market is expected to demand.

"To be truly sustainable," says Mahmood, "the hydrogen we use must be 100% carbon-free across the entire production life cycle and must not cut into the world’s precious freshwater reserves. Our method to produce hydrogen straight from seawater is simple, scaleable and far more cost-effective than any green hydrogen approach currently in the market. With further development, we hope this could advance the establishment of a thriving green hydrogen industry in Australia."
The team will move to scale up as research continues. The next step is to build a prototype electrolyzer system running a stack of these catalyst sheets, to produce large quantities of hydrogen and start optimizing system-level efficiency at scale. Mahmood believes this technology could help achieve the Australian government's goal of green hydrogen production at AU$2/kg (US$1.40/kg), the level at which it becomes cost-competitive with dirty hydrogen produced using fossil fuels.
At the end of the day, that's the key metric here; for companies to invest heavily in large-scale green hydrogen production, they need to know they can do it profitably, and compete with other hydrogen and fuel sources. Let's see how it all shakes out, but we certainly hope some of these seawater-splitting innovations work as well on a balance sheet as they do in the lab.
Check out a short video below.
The paper is open access in the journal Small.
Source: RMIT






