A new announcement from vaccine company Valneva indicates its experimental COVID-19 vaccine is effective at neutralizing the Omicron variant. The vaccine is at the tail-end of Phase 3 trials and is the first inactivated whole-virus vaccine to be trialed in Europe.
French company Valneva’s vaccine candidate is dubbed VLA2001 and it works a little differently to most currently approved COVID-19 vaccines.
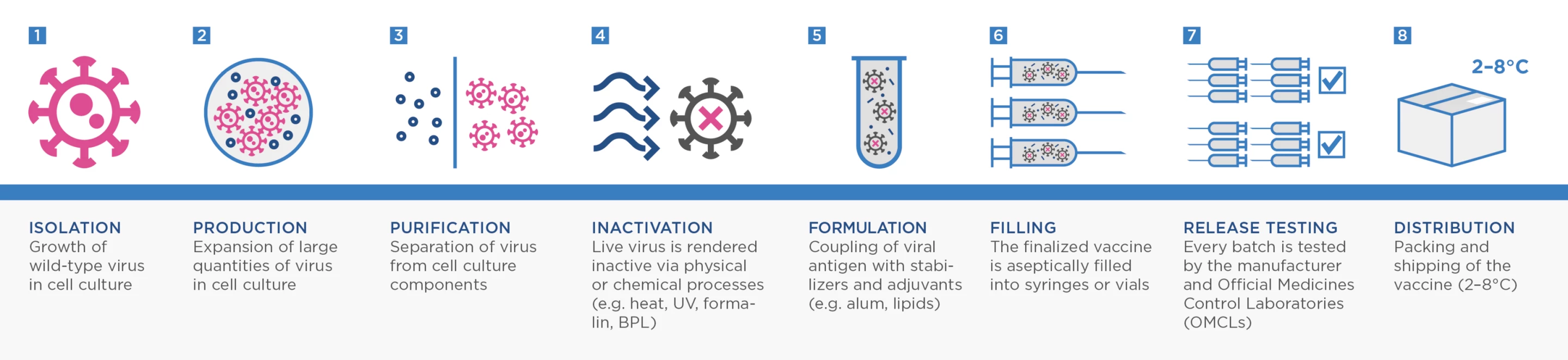
Most current vaccines focus on presenting a person’s immune system with the spike protein of SARS-CoV-2. But VLA2001 is what is known as an inactivated whole-virus vaccine. These vaccines actually contain complete copies of the whole virus grown in a lab and then inactivated (or "killed") using chemicals or heat.
This process is one of the more traditional ways to make a vaccine. It has been utilized successfully for decades, going back to Jonas Salk’s landmark polio vaccine and has been more recently used to develop annual influenza vaccines.
Adam Taylor, a researcher from Australia’s Griffith University, explained last year that there is hope inactivated whole-virus vaccines could be more effective against SARS-CoV-2 variants because they help the body learn how to target more viral antigens beyond just the spike protein.
“This type of inactivation is expected to preserve the structure of the viral proteins, as they would occur in nature,” Taylor explained in a piece for The Conversation. “This means the immune system will be presented with something similar to what occurs naturally, and mount a strong immune response.”
VLA2001 is currently deep in Phase 3 human trials. Ongoing trial data has been consistently supplied to regulatory bodies in Europe and approvals are expected to come over the next few months.
The latest announcement from Valneva reports on lab studies looking at how effective antibodies induced by the vaccine are at neutralizing both the Delta and Omicron SARS-CoV-2 variants. The research isolated antibodies from human trial subjects who had been given three shots of VLA2001.
All samples tested were effective at neutralizing the original strain of SARS-CoV-2 and the Delta variant. Against Omicron the research found 87 percent of samples presented neutralizing antibodies.
The findings are certainly promising, however, it is still unclear what kind of real-world protection may be generated by VLA2001 in the face of Omicron.
Valneva is not the first inactivated whole-virus vaccine to be developed for COVID-19. In fact, China’s Sinovac and Sinopharm vaccines use similar technology and have already been administered to billions of people.
However, recent research has indicated current whole-virus vaccines may not be performing well against the Omicron variant. Studies have found immune responses to Omicron after two shots of many currently available whole-virus vaccines are “sub-optimal.”
What this means for Valneva is unclear. It could be that whole-virus vaccines require a standard three-dose protocol to be effective, or maybe Valeneva’s unique combination of adjuvants added to the vaccine help prompt better immune responses. Or possibly these lab studies measuring antibody neutralization simply don’t translate into real-world protection against Omicron.
What we do know is that Valneva’s vaccine demonstrates a good safety profile and should be relatively easy to manufacture. Over the coming months its approval should help amplify vaccine supplies to countries struggling for doses and offer well-vaccinated countries new options for booster programs.
“This is very encouraging data and adds to the positive Phase 3 data,” said Clive Dix, former lead on the UK Vaccine Taskforce. “Hopefully this vaccine will be approved soon and should become an important vaccine in both this pandemic in countries still behind the curve and as a vaccine for boosting during the winter of 2022/23.”
Source: Valneva