Researchers at TU Wien have developed a new way to grow cartilage from stem cells and guide it into basically any shape required. The breakthrough could lead to better ways to patch up injuries.
Cartilage is the rubbery tissue in joints that acts like a cushion to stop bones rubbing against each other. But as important as it is, it has almost no regenerative ability in adults, so when it’s damaged due to injury or just decades of wear-and-tear, it can lead to painful chronic conditions like osteoarthritis.
Scientists are investigating how to make artificial replacement materials, but it’s looking like nothing will be able to top nature’s own stuff. So, another potential solution is to find ways to regenerate natural cartilage using stem cells, but this brings up other issues, including how to get them to grow in the right shape, with clumps of these stem cells often changing shape or shrinking.
For the new study, the TU Wien team developed a technique that can grow samples of cartilage into basically any shape needed, which they demonstrated by forming it into the university’s logo. The key innovation isn’t so much the stem cells but the container they’re put in – tiny, hollow, 3D-printed “spheroids” that can be connected to each other like building blocks, providing a scaffold for the cartilage stem cells inside.
“Under the microscope, you can see very clearly: neighboring spheroids grow together, the cells migrate from one spheroid to the other and vice versa, they connect seamlessly and result in a closed structure without any cavities - in contrast to other methods that have been used so far, in which visible interfaces remain between neighboring cell clumps,” said Oliver Kopinski-Grünwald, an author of the study.
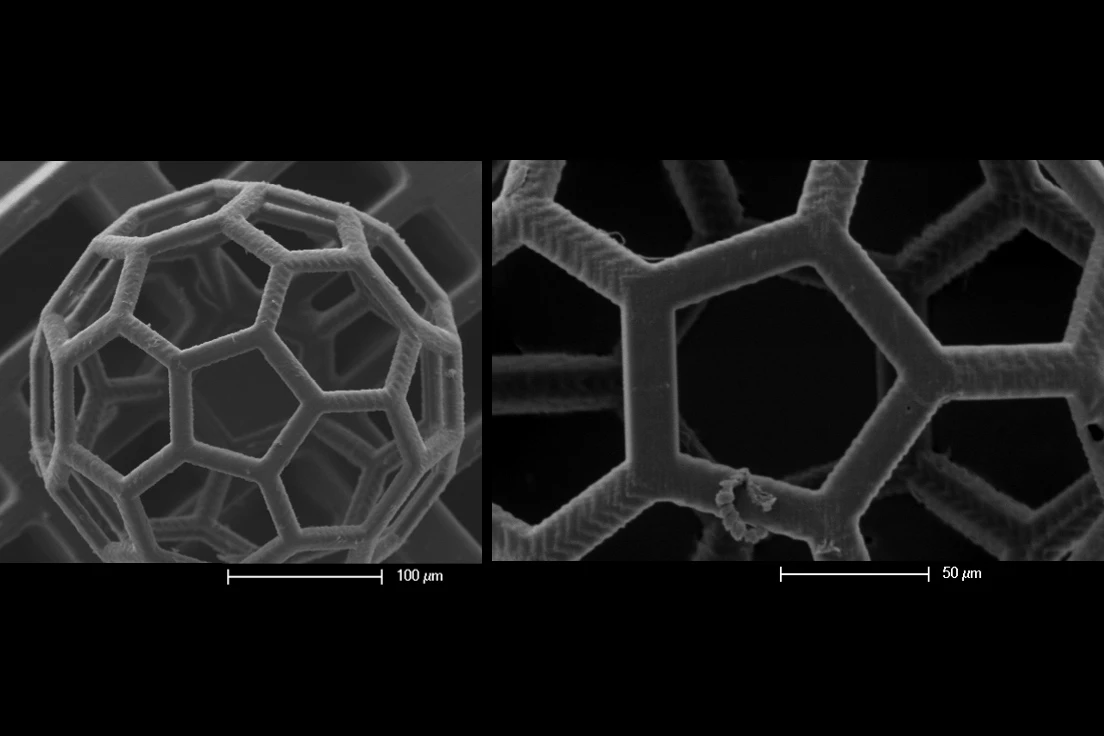
The spheroids are made of a biocompatible plastic material, which provides stability and structure in the beginning, but breaks down over a few months, leaving behind the tissue in the desired shape. The team says that this could make for more effective, customizable cartilage replacements.
“An initial goal would be to produce small, tailor-made pieces of cartilage tissue that can be inserted into existing cartilage material after an injury,” said Kopinski-Grünwald. “In any case, we have now been able to show that our method for producing cartilage tissue using spherical micro-scaffolds works in principle and has decisive advantages over other technologies.”
Cartilage is an attractive target for this kind of work – not just because it’s a tissue that people often have problems with, but because it’s relatively simple and doesn’t require blood vessels. If the challenge of incorporating blood vessels into these custom-grown tissues can be overcome, the researchers say the technique could be adapted to work with other tissues like bone.
The research was published in the journal Acta Biomaterialia.
Source: TU Wien





