Researchers have, for the first time, observed a mechanism used by cancer cells to resist the effects of chemotherapy. The findings could be used to develop targeted drugs to help override it and make chemotherapy more effective.
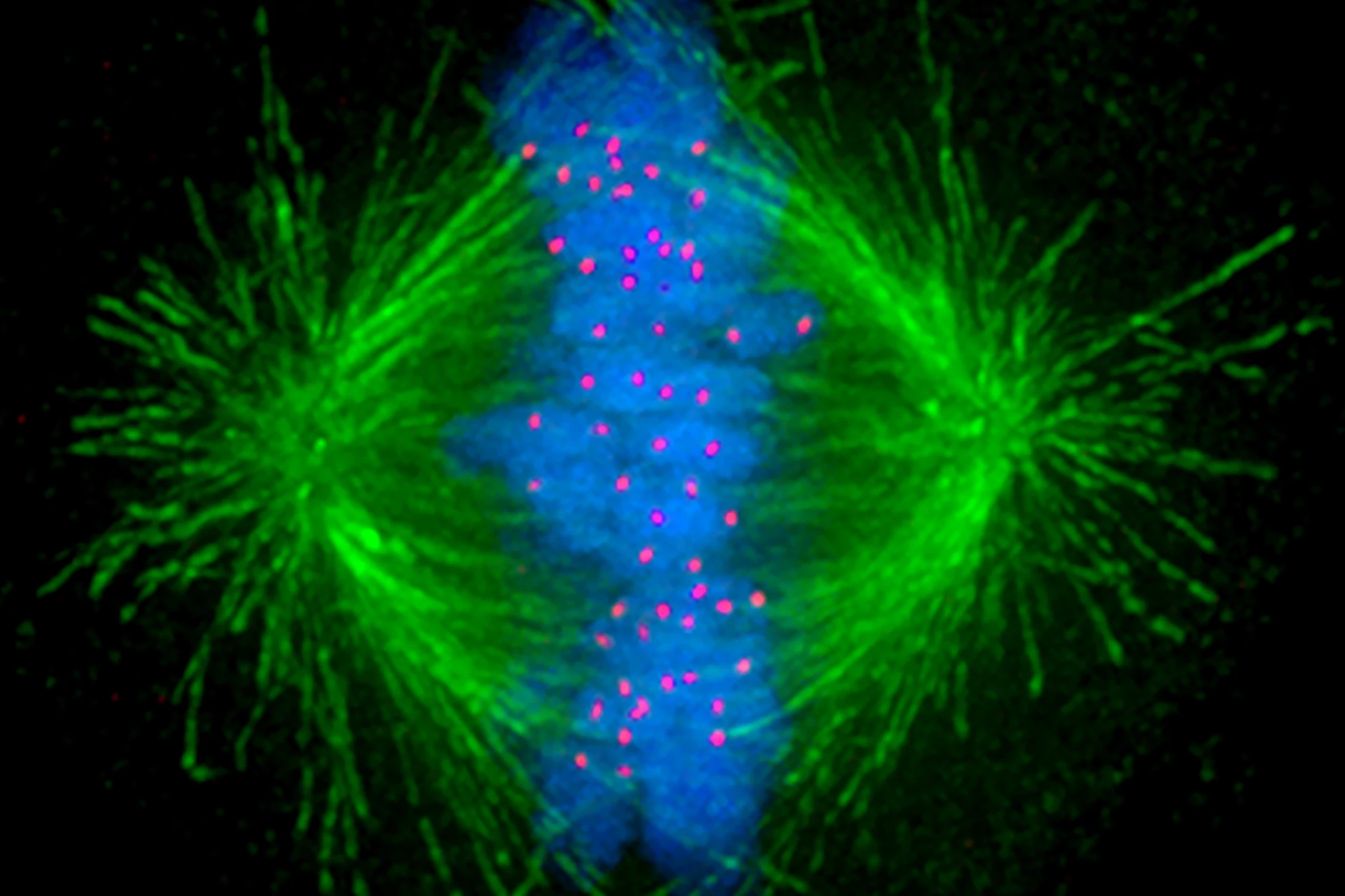
When cells divide, tiny, tube-like structures inside the cell called microtubules act like mechanical arms, separating genetic material and ensuring the successful production of daughter cells. Given the increased rate at which cancer cells divide compared to normal cells, anti-microtubule chemotherapy drugs target these structures to inhibit the cancer’s growth.
But cancer cells are crafty and have developed ways of ensuring the treatment isn’t always effective. Now, for the first time, researchers at the University of New South Wales (UNSW) Sydney have observed a mechanism that cancer cells employ to resist the effects of chemotherapy.
“The anti-microtubule chemotherapy usually fragments the mechanical arms into multiple hubs that pull the chromosomes in multiple directions rather than the normal two,” said Peter Gunning, corresponding author of the study. “The resulting chaos prevents proper separation of the chromosomes to the two daughter cells and induces apoptosis or programmed cell death.”
The researchers found that the cancer cells use a nifty technique to allow them to continue dividing and thus avoid the effects chemotherapy is designed to induce.
“We discovered that cancer cells use the mechanical force supplied by the edge of the cell called the cell cortex to overcome the impact of commonly used chemotherapy that blocks the ability of the cell to separate the chromosomes during cell division,” Gunning said.
The cancer cells activate a signal when the microtubules fragment that causes the arms to reach out to the cell’s edge and pull on the cortex to bring the fragments back together.
“This allows the arms to stabilize and generate the force necessary to physically grab and pull the chromosomes into each daughter cell and ensure the cancer cell multiplies,” said Gunning.
The researchers suspected the mechanism existed after noticing a specific microtubule-targeting drug used to treat neuroblastoma, a childhood cancer, enhanced the effects of chemotherapy. But, at the time of their previous study, imaging was not advanced enough to confirm their suspicions.
“We needed good imaging of the cancer cells as they go through cell division to visualize what’s happening to the chromosomes, the microtubules and the architecture of the cells in real-time,” said Gunning. “It was quite surprising to us because we did not expect this mechanism of the cancer cell to be used in this way to overcome the cancer therapy, but we could see it happening before our eyes.”
High doses of chemotherapy are generally effective at stopping cancer cells from dividing. However, at lower doses – when, say, a patient develops chemotherapy toxicity and the dose needs to be reduced – the cells can take advantage of this innate survival mechanism, something the researchers think is a fundamental component of cell biology.
“We think it’s a fallback mechanism that evolved to allow any cell to overcome a small amount of microtubule disruption and ensure it can survive,” Gunning said. “It just so happens cancer cells use it to sidestep the anti-microtubule chemotherapy.”
The researchers are focused on developing drugs that work in combination with current chemotherapeutic agents to shut off the resistance mechanism.
“By attacking the force-generating machinery built by the cancer cells, we expect that we will be able to allow the cancer therapy to do its job much more effectively,” said Gunning. “In practical terms, we have established a company that will allow us to develop the drugs needed to attack this rescue mechanism, enabling the anti-microtubule chemotherapy to act more effectively and hopefully improve patient outcomes.”
The study was published in the journal Current Biology.
Source: UNSW Sydney