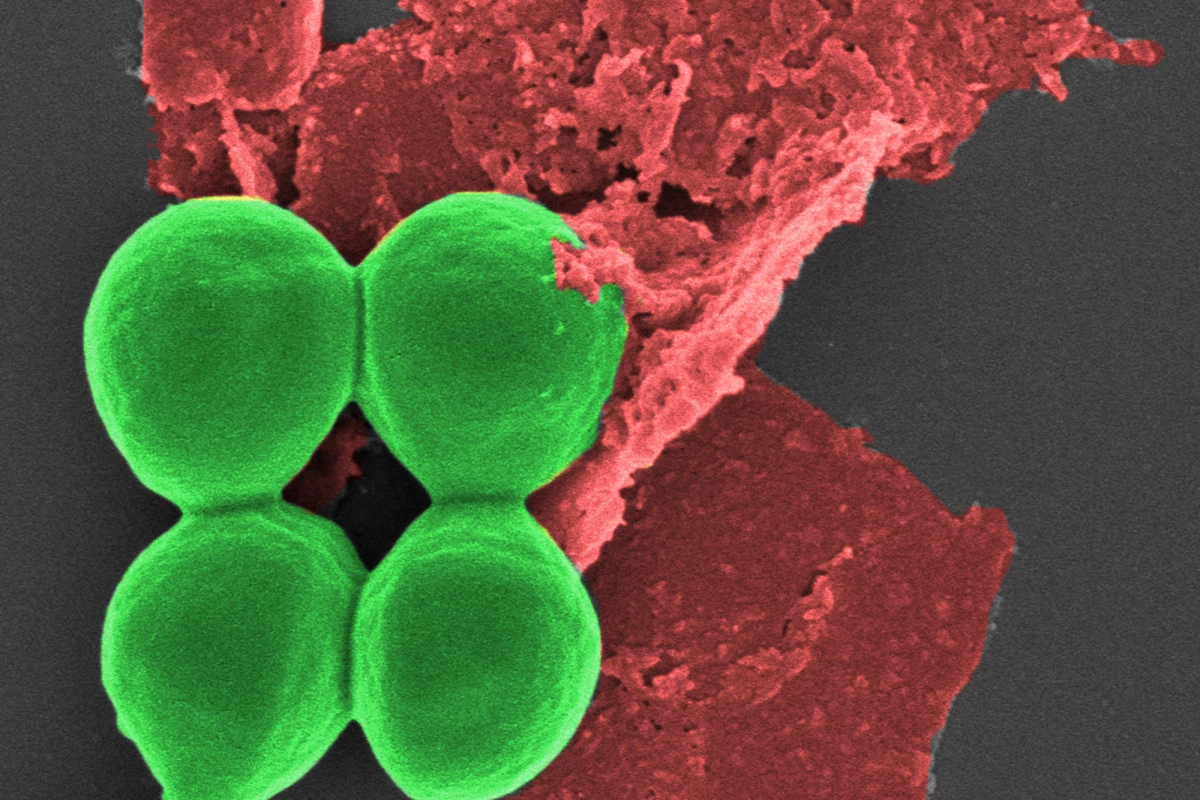
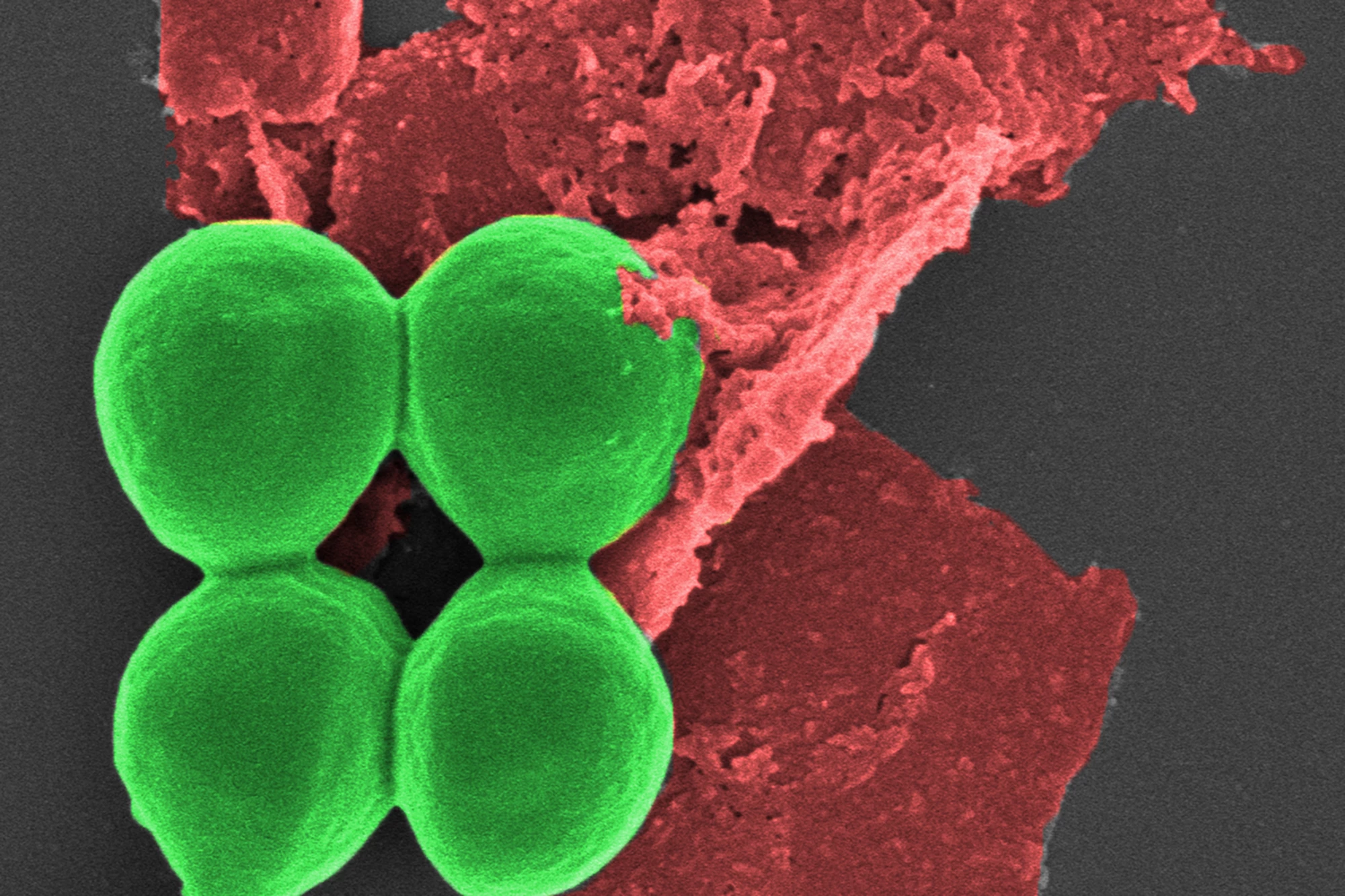
Researchers have found that using nano-sized flakes of black phosphorus on wounds infected with drug-resistant superbugs not only kills the pathogens, but also promotes wound healing. More than a coating, the innovative antimicrobial can be incorporated into common materials such as dressings, gels, and plastics.
With the challenge posed by the rise of superbugs, we need to come up with novel ways of tackling wound infections. That need is thrown into sharp relief when you consider that around 70% of bacteria have developed resistance to at least one common class of antibiotic, and since 2000, only five new classes of antibiotics have been discovered.
Recently, researchers from RMIT University in Australia proposed a novel, drug-free method of preventing post-op infections in people receiving titanium implants. Now, they’ve teamed up with researchers from the University of South Australia to develop another innovation, using nano-sized flakes of black phosphorus to tackle wound infection caused by superbugs.
“Superbugs – the pathogens that are resistant to antibiotics – are responsible for massive health burdens, and as drug resistance grows, our ability to treat these infections becomes increasingly challenging,” said Aaron Elbourne, one of the study’s co-authors.

Black phosphorus has recently been identified as an effective antimicrobial agent. It’s the most stable physical form of phosphorus and consists of 2D layers of phosphorus (called ‘phosphorene’), the same way that graphite comprises many graphene layers. In their previous work, the researchers demonstrated how black phosphorus arranged in nano-thin layers killed microbes by its unique ability to produce reactive oxygen species.
“As the nanomaterial breaks down, its surface reacts with the atmosphere to produce what are called reactive oxygen species,” said Sumeet Walia, a co-author of the study. “These species ultimately help by ripping bacterial cells apart.”
In the current study, the researchers tested the safety and efficacy of using black phosphorus nanoflakes (BPNFs) on common bacteria, including drug-resistant S. aureus (‘golden staph’), P. aeruginosa, and E. coli.
S. aureus treated with BPNFs showed a 62% loss of cell viability within two hours, with an 80% loss of viability after six hours. After 24 hours, over 99% of bacteria were killed. A similar trend was seen with P. aeruginosa, with BPNFs causing over 80% bacterial death after 24 hours. Not only did the BPNFs destroy the bacteria without damaging other cells, but they also self-decomposed after the infection threat had been eliminated.
“Our antimicrobial nanotechnology rapidly destroyed more than 99% of bacterial cells – significantly more than common treatments used to treat infections today,” Walia said.
When the researchers tested the effectiveness of BPNFs as against ciprofloxacin, a commonly used broad-spectrum antibiotic, on mouse wounds, they found that both were comparably effective at clearing S. aureus.
BPNFs also demonstrated enhanced wound healing and tissue regeneration at a macro- and microscopic level, compared to controls. Daily treatment with BPNFs over seven days produced an 80% wound closure, with no evidence of redness or skin breakdown.
The researchers concluded that the observed improvement in the degree of re-epithelialization – the creation of a barrier between wound and environment – suggested that BPNFs promote wound healing even when wounds are infected with a highly resistant S. aureus bacteria. While the antimicrobial properties of black phosphorus are known, its wound healing properties are not well documented.
“This is exciting as the treatment was comparable to the ciprofloxacin antibiotic in eradicating wound infection and resulted in accelerated healing, with wounds closing by 80% over seven days,” said Zlatko Kopecki, corresponding author of the study. “We urgently need to develop new alternative non-antibiotic approaches to treat and manage wound infection. Black phosphorus seems to have hit the spot and we look forward to seeing the translation of this research towards clinical treatment of chronic wounds.”

The appeal of BPNFs, say the researchers, is that they can be incorporated into a range of materials.
“The beauty of our innovation is that it is not simply a coating – it can actually be integrated into common materials that devices are made of, as well as plastic and gels, to make them antimicrobial,” said Walia.
The research team is looking to collaborate with industry partners to develop and prototype the technology.
“If we can make our invention a commercial reality in the clinical setting, these superbugs globally wouldn’t know what hit them,” Elbourne said.
The study was published in the journal Advanced Therapeutics.
Source: RMIT University