Testing for protein clumps in spinal fluid is the conventional way of detecting Alzheimer’s disease, but it doesn’t provide information about the disease's stage. In a new study, researchers were able to stage the disease using atomic force microscopy to visualize the size and shape of these clumps, which may provide a way of detecting the debilitating disease earlier.
Cerebrospinal fluid (CSF) is a clear fluid that surrounds the brain and spinal cord, providing nutrients and acting as a watery cushion to protect these structures from injury.
CSF can be analyzed to measure the level or concentration of different substances and cells to diagnose conditions that affect the central nervous system. CSF analysis is key to diagnosing neurodegenerative disorders, including Alzheimer’s disease. Of particular interest are two pathological proteins: tau, which causes ‘tangles’ in the brain cells (neurons), and amyloid beta, which causes neuronal plaques; both are hallmarks of the disease.
Swiss Federal Laboratories for Materials Science and Technology (EMPA) researchers studied aggregated tau and amyloid beta protein bundles (called fibrils) in the CSF of people with Alzheimer’s disease in hopes of developing a way of detecting Alzheimer’s earlier.
Current methods of detecting Alzheimer’s disease analyze CSF for the presence and quantity of these disease-causing proteins. As biomarkers, they’re of limited use as they can’t provide information on the protein’s size and shape (morphology), which is key to determining how advanced the disease is.
In the current study, the researchers used atomic force microscopy (AFM) to visualize the protein fibrils in the CSF of 33 people with varying degrees of cognitive impairment, from mild to advanced dementia.
AFM is a powerful technique for imaging almost any surface type, including biological samples. Using a very sharp tip attached to a silicon probe, images of the sample are taken line by line. Importantly for this study, AFM enabled the researchers to inspect the proteins in a nanometer range without destroying them.
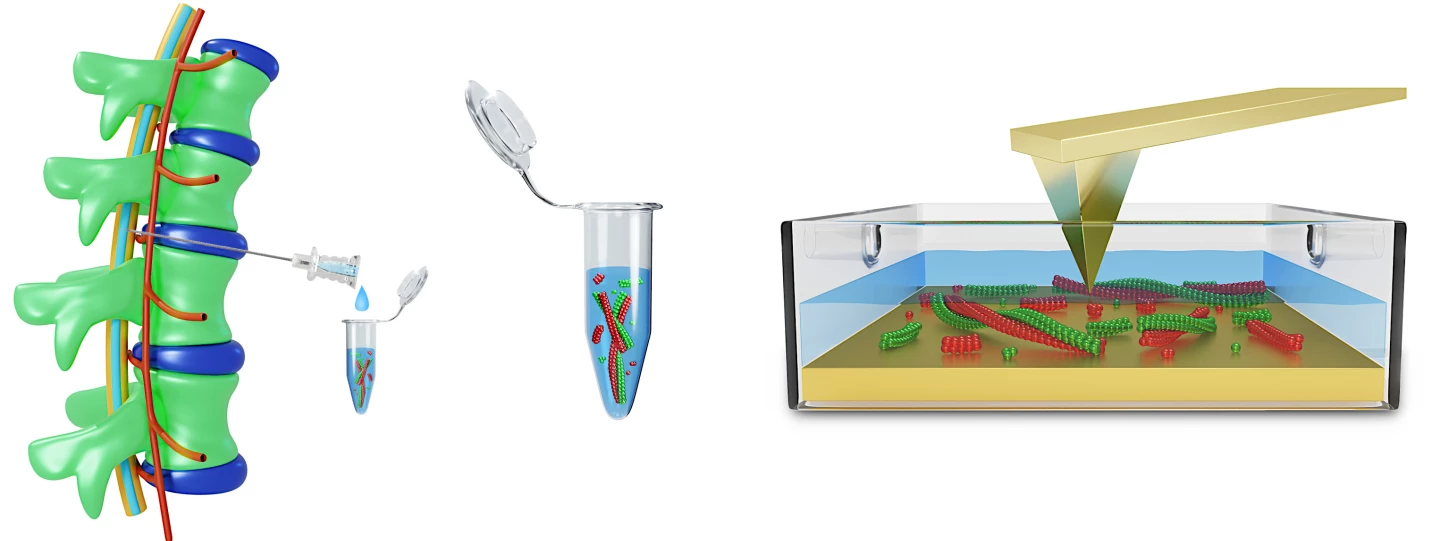
By analyzing the proteins’ size, structure and shape, and how they clump together, the researchers could link different morphologies to a particular stage of Alzheimer’s disease.
“While only short proteins fibrils, about 100 nanometers in length, were found in people at an early stage of the disease, fibrils with multiples of this length – reaching several micrometers – appeared in later stages of the disease,” said Peter Nirmalraj, lead author of the study.
The CSF of healthy people contained no or even shorter fibrils than those seen in people with dementia.
The study’s findings further expand our knowledge about how Alzheimer’s disease develops and provides a new, more precise biomarker to gauge how far the disease has progressed.
The researchers say that using AFM to observe the characteristics of protein clumps in the CSF can be used in conjunction with existing methods for detecting Alzheimer’s disease.
“AFM technology has the potential to complement conventional biomarker tests and improve the early detection of Alzheimer’s,” Nirmalraj said.
They say their findings may lead to the development of new, more effective drugs to treat the as-yet incurable disease.
The study was published in the journal Communications Biology.
Source: EMPA





