A Phase 3 clinical trial of the drug donanemab has shown it can significantly slow cognitive and functional decline in people with early-stage Alzheimer’s disease, compared with existing treatments.
Phase 3 trials use large groups to compare a new drug treatment with an existing one to determine which treatment works better (efficacy) and to learn more about the new drug's side effects.
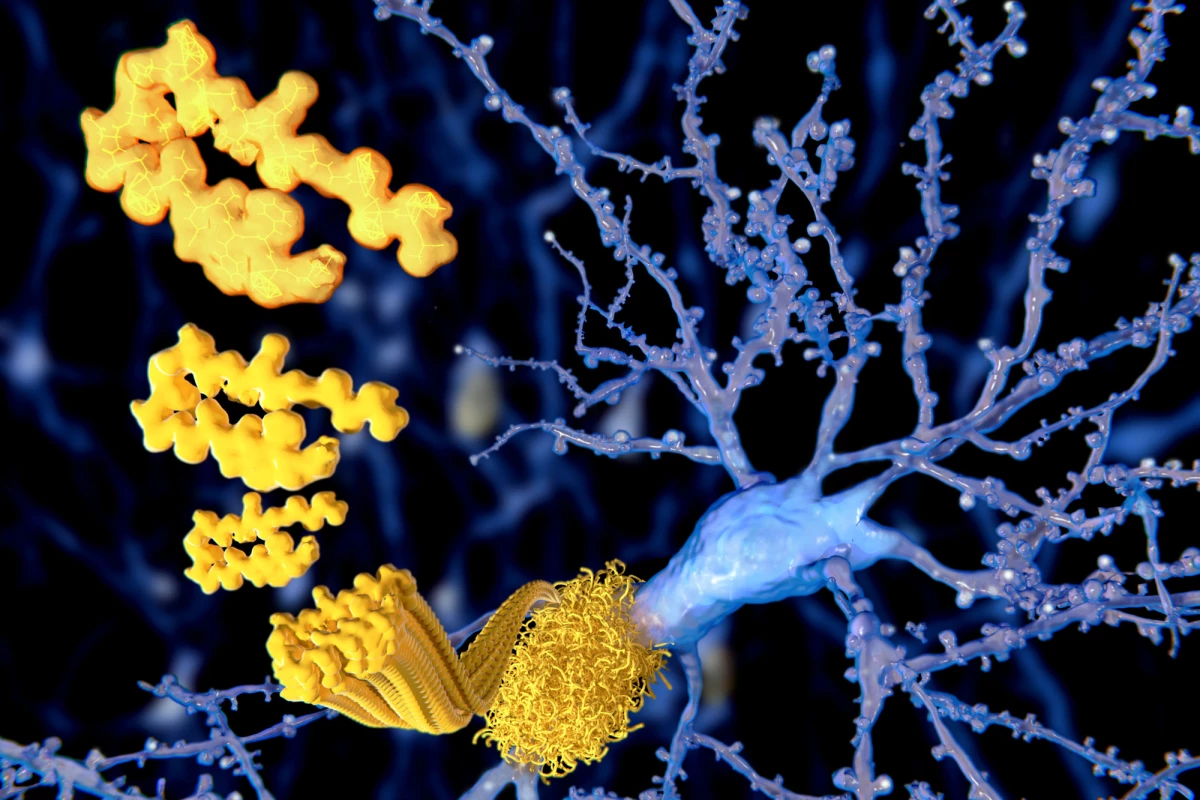
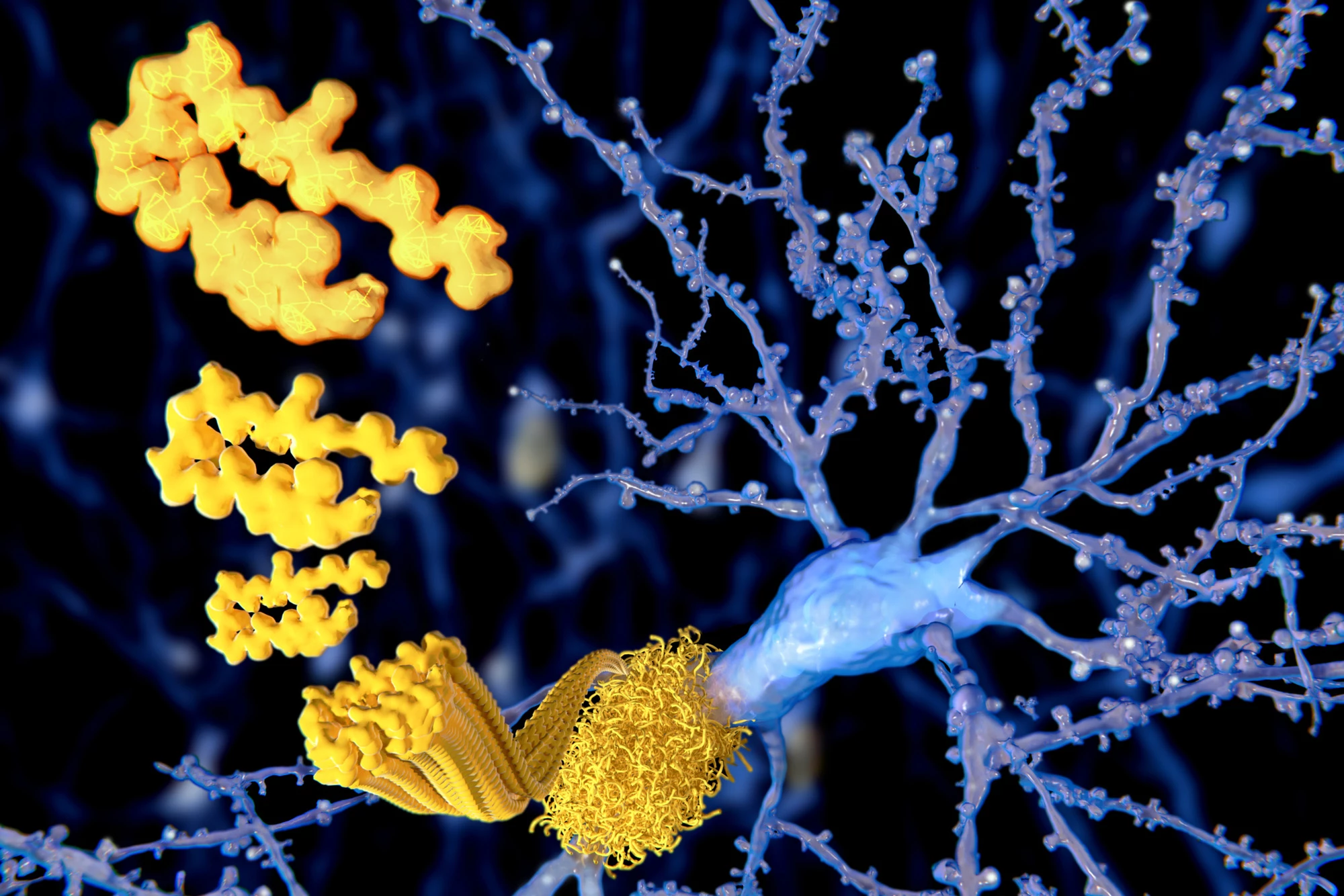
Donanemab is an immunotherapy drug that targets the protein amyloid beta, a hallmark of Alzheimer’s disease. When amyloid beta accumulates in the brain, it creates sticky plaques that damage brain cells (neurons). A monoclonal antibody, donanemab clears away the plaque by targeting a specific kind of amyloid beta – pyroglutamate-modified amyloid beta.
The trial, known as TRAILBLAZER-ALZ 2, evaluated the safety and efficacy of donanemab on 1,182 people with symptoms of early Alzheimer’s disease, such as mild cognitive impairment. It was compared with lecanemab, another monoclonal antibody drug that targets amyloid beta. While both drugs are given intravenously, donanemab is given every four weeks, whereas lecanemab is given every two.
Participants in the current trial were categorized according to the level of the protein tau, which is predominantly found in neurons, can create ‘tau tangles’, and is another hallmark of Alzheimer’s.
They were scored using the Clinical Dementia Rating-Sum of Boxes (CDR-SB) tool, which evaluates cognition and function in neurodegenerative diseases. Their ability to perform activities of daily living was assessed using the integrated Alzheimer’s Disease Rating Scale (iADRS), a measure of their ability to undertake activities such as managing finances, driving, and conversing about current events.
The trial produced some promising results. Assessing the participants’ iADRS scores, the cognitive and functional decline of those who received donanemab was slowed by 35% compared to the placebo group.
At the one-year mark, 47% of participants who took donanemab showed no decline in the CDR-SB, compared to 29% who were given the placebo. And participants on donanemab demonstrated a 39% lower risk of progressing to the next stage of the disease.
The drug significantly reduced brain amyloid plaque levels, as observed using positron emission tomography (PET) brain scans. 34% of participants with intermediate levels of tau protein achieved amyloid clearance at six months; 71% at 12 months. However, despite donanemab clearing amyloid plaques, the disease was not halted completely.
The trial’s results have created a cautious buzz in the medical community.
“The results of this study are very encouraging for patients with Alzheimer’s disease,” said Bruce James Brew, consultant physician and neurologist at the University of New South Wales, Australia. “While the full results are yet to be published, the data that have been released show it significantly slows progression of Alzheimer’s. However, it is definitely not a cure for Alzheimer’s.”
The drug’s risk of potentially serious side effects, such as brain swelling and bleeding, has raised concerns. Swelling was seen in 24% of treated participants, with 6.1% experiencing mild-to-moderate symptoms. Microhemorrhages were seen in 31.4% of treated participants compared with 13.6% in the placebo group. Three participants died during the trial.
“Donanemab is not without potentially significant risk,” said Lyndsey Collins-Praino, head of the Cognition, Ageing and Neurodegenerative Disease Laboratory (CANDL) at the University of Adelaide. “As treatment was completed once plaque clearance was achieved, questions also remain about long-term benefits, given that cognitive/functional decline is not dependent on plaque pathology alone. While I am cautiously optimistic, key questions remain.”
Based on the results of the clinical trial, Lilly hopes to apply for FDA approval for donanemab later this year.