The field of nanomedicine is progressing rapidly and in recent years has opened up all kinds of exciting possibilities, with scientists showing how tiny particles could be deployed to deliver drugs, fight inflammation and prevent heart attacks. A new study has demonstrated how they can be used as a type of mechanical drug inside cells to impair function and cause their demise, which could be used to target cancer while leaving healthy cells unharmed.
The discovery is the handiwork of scientists at the Spanish National Research Council, who set out to better understand the way mechanical cues can determine the fate of individual cells. The cytoskeleton that gives cells their shape and structure combines with the membrane to form a mechanical system, which can alters a cell's function when subjected to physical forces.
Certain therapeutics, such as the chemotherapy drug Paclitaxel, are designed to target and alter this mechanical function and drive cell death. Better understanding of these delicate systems could open up yet more possibilities when it comes to dictating the function of certain cells, and lead to targeted and highly potent therapies that destroy cancer cells from the inside.
To explore these possibilities, the researchers designed a type of silicon nanochip to destabilize cell mechanics during a type of cellular division called mitosis.
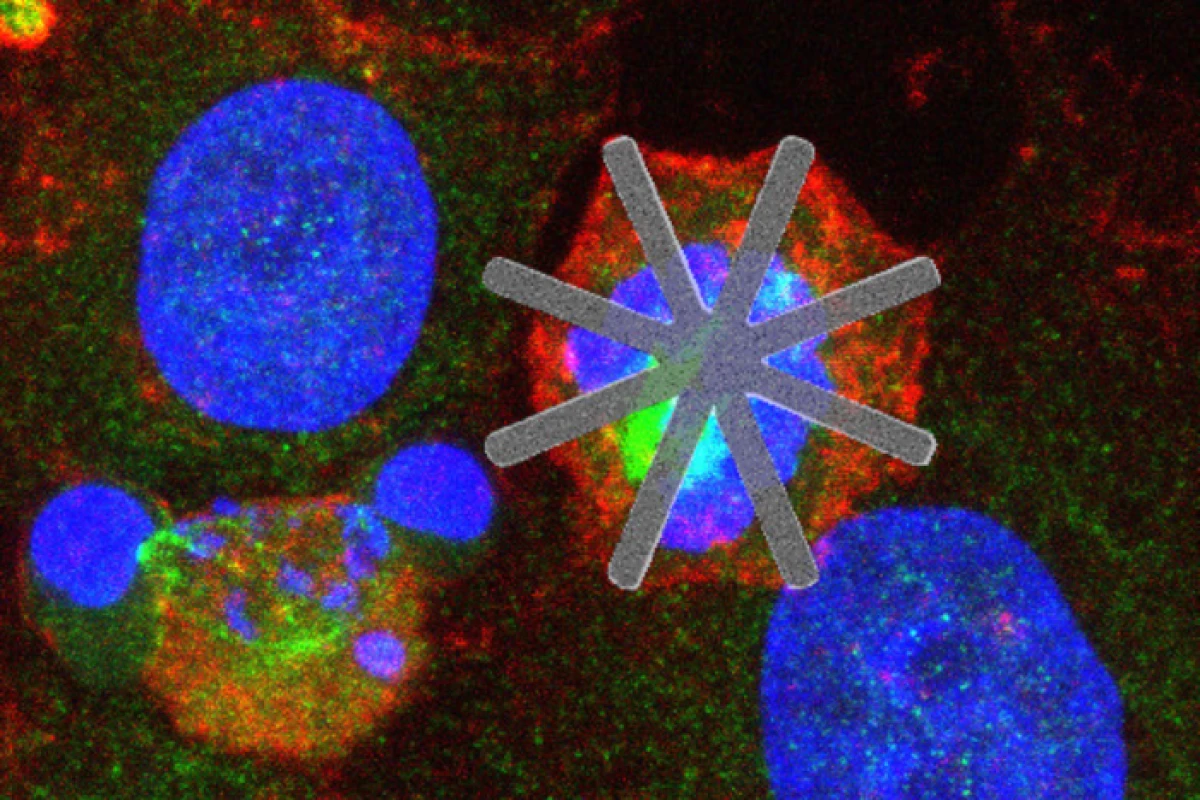
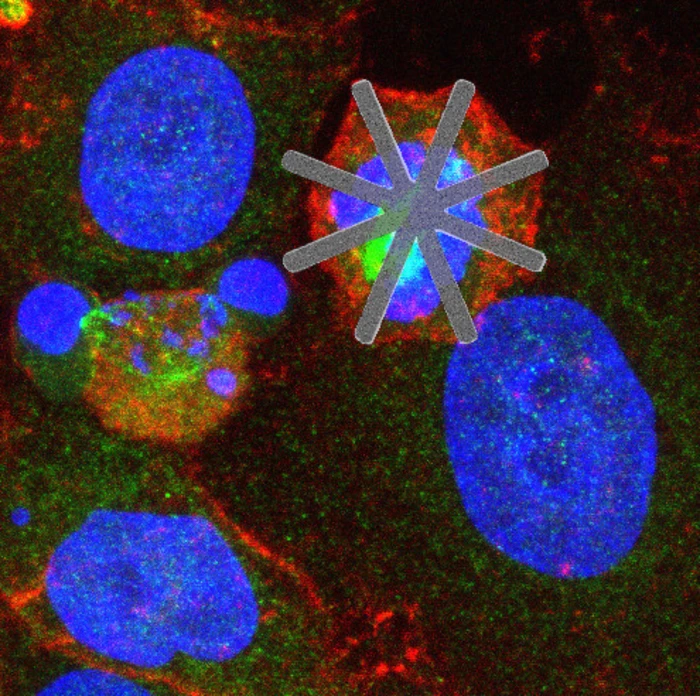
"The devices can be designed with a high control of their shapes and dimensions at the scale of microns and nanometers," explained project coordinator José Antonio Plaza. "Specifically, the manufactured devices are star-shaped, with a 22-micrometer diameter and thicknesses ranging from 50 to 500 nanometers. They are made of silicon and their star-shaped geometry resembles a mesh of nanofibers."
Working with cervical cancer cells in the lab, the scientists incubated them together with their star-shaped chips and found they were incorporated into the cell at a high rate, "typically by catching the proximal arm of the star." Around 90 percent of the cells that internalized the devices showed hallmarks of altered cell cycles, with delayed or blocked cell division occurring in around half. "This suggests that cell death was a direct consequence of star device internalization and, more often, subsequent MACC (mechanically altered cell cycle)," the team writes in their paper.
"Preventing cell division or slowing it down by means of a mechanical obstacle can cause cell death and can be key in future medical treatments," said study author Teresa Suárez. "Our research shows that these tools could be an innovative starting point for the study of different diseases, such as cancer."
By experimenting with chips of differing thicknesses, shapes, sizes and stiffness, the team was also able to show how tweaks to the design could bring about different results. For instance, ones shaped as larger discs weren't so readily taken up by the cells, but profoundly altered the cell cycle and induced death in a high percentage of cells when they did make it inside. In this way, the tiny chips serve not just as a potential weapon against cancer and disease, but a new platform to study cellular mechanics.
"The capacity to manufacture millions of silicon chips of rigorously accurate size and shape will allow the design of new tools that will facilitate the exploration of cellular mechanics from innovative perspectives, as well as to contribute to the knowledge of intracellular processes," adds study author María Isabel Arjona.
The research was published in the journal Advanced Materials.