Bacteria may be great at developing resistance to our best drugs, but there are some things they can’t evolve their way out of – like tiny drills ripping them open. Researchers at Rice University have now demonstrated their next generation molecular drills that can be activated by blue light and could revolutionize the fight against superbugs.
Bacteria adapt quickly to changes in their environment, and unfortunately that includes antibiotics. Some of the bugs will naturally have mutations that allow them to survive the onslaught, then pass those genes on to future generations. Over time, that resistance builds up to the point that most of our once-powerful antibiotics are failing, which could eventually lead to a “new dark age of medicine” where basic infections become lethal again.
But while bacteria can develop resistance to chemical attacks, they can’t defend against physical attacks. As such, scientists are investigating killing superbugs using jagged surfaces, black phosphorus coatings, liquid-metal shredders, micro-traps, “poisoned arrows” and molecular drills.
It’s that last one that the Rice team has now built on. These molecules are designed to have moving rotors that, when activated by light, spin between two and three million times per second, making them effective drills. When that motion is directed onto bacteria, it can pierce their outer membranes and spill their insides, either killing the cell outright or providing an opening for antibiotics to get in and finish the job.
Now the Rice researchers have made a few tweaks to the drills. Previous iterations were activated by ultraviolet light, but the new ones now run on blue light, which is harmless to human cells and already has some antibacterial properties of its own. The team also made some modifications that guide the drills towards bacteria better, by homing in on the negative charge of their membrane.
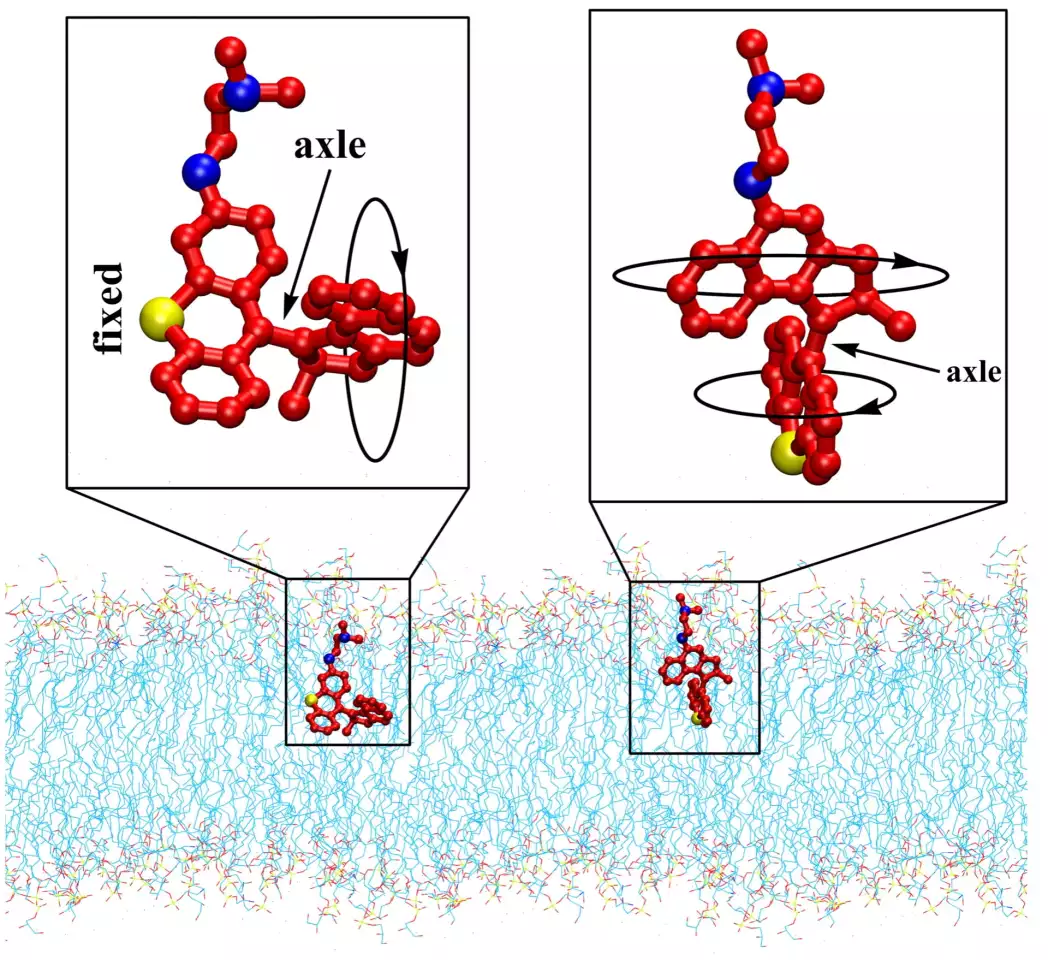
The team tested six variations of the drills, and found that all of them were able to punch holes in the membranes of both major groups of bacteria – gram-negative and gram-positive – reducing their levels below detectable amounts in a matter of minutes. The drills were also effective at busting through biofilms – protective shields that bacteria build around their colonies – and killing persister cells, which are the stragglers that survive antibiotics by going dormant.
While the drills alone were enough to kill the majority of the bacteria, adding antibiotics made the treatment even more powerful. This worked even when the bugs had already developed resistance to those specific drugs, as it seems the drills help bypass the evolved defenses. This means that the drills might not just be a promising new treatment on their own, but could extend the useful life of existing antibiotics.
The team says that the next steps are to link bacteria-specific peptide tags to the drills, which will help them hunt down targeted strains of bacteria more precisely, while leaving human cells alone.
The research was published in the journal Science Advances.
Source: Rice University





