Adding chlorine is one of the most common methods of disinfecting drinking water, but just how safe is it? Researchers from Johns Hopkins have now found evidence that reactions between chlorine and natural compounds in water may produce previously-unknown toxic byproducts.
It’s well known that chlorine is a powerful disinfectant, killing bacteria, viruses and other microbes effectively. As such, it’s credited with drastically stemming the tide of waterborne disease such as cholera and typhoid, after it became widely used to treat drinking water in the early 20th century.
But adding chlorine isn’t without its own problems. The chemical reacts with compounds called phenols that are naturally found in water, creating potentially-harmful byproducts. But as the World Health Organization (WHO) says in a report (PDF, page 6), “the risks to health from these byproducts are extremely small in comparison with the risks associated with inadequate disinfection.”
But now, the Johns Hopkins researchers have found signs of new byproducts that have until now gone undetected. The team suspected that current methods of analyzing the chemistry of drinking water may miss some of these byproducts, so they tested another technique.
The researchers used N-α-acetyl-lysine, an amino acid that’s often used in toxicology to detect harmful molecules known as reactive electrophiles. The team added this amino acid to water that had first been treated with chlorine the same way that drinking water usually is at large scales. They then left it to sit for a full day before analyzing it using mass spectrometry.
And sure enough, the researchers detected two related compounds: 2-butene-1,4-dial (BDA), and chloro-2-butene-1,4-dial (BDA with chlorine). These are known to be toxic and carcinogenic, and have never been detected in drinking water before.
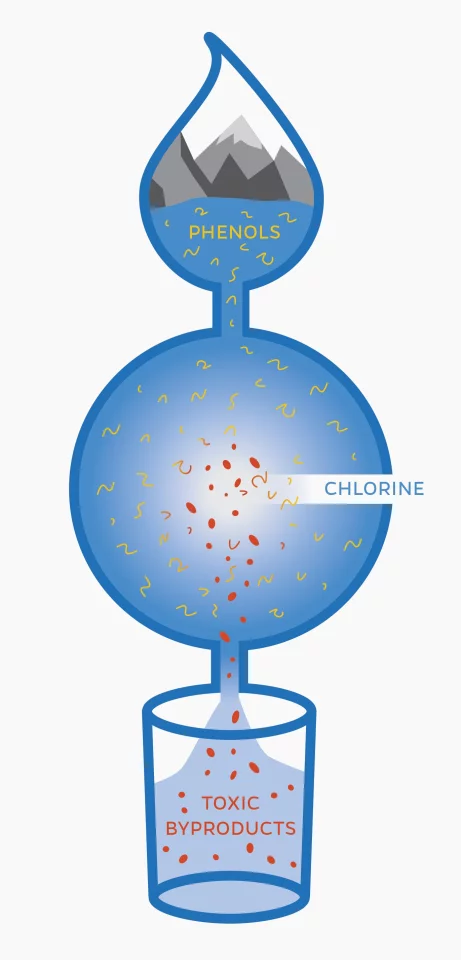
That said, the team points out that they still haven’t been found in actual drinking water yet – the lab tests just show that they could be produced under those conditions. There’s also the question of whether these compounds are present in high enough concentrations to pose any actual threat to health. And even then, there’s still the tradeoff that preventing waterborne illnesses is likely a much greater good.
"There's no doubt that chlorine is beneficial; chlorination has saved millions of lives worldwide from diseases such as typhoid and cholera since its arrival in the early 20th century," says Carsten Prasse, lead author of the study. "But that process of killing potentially fatal bacteria and viruses comes with unintended consequences. The discovery of these previously unknown, highly toxic byproducts, raises the question how much chlorination is really necessary."
Alternate methods of disinfecting water, such as ozone, UV light or filtration, keep waterborne disease at bay in many countries around the world without producing toxic byproducts. The team says that it may be better to put these into even wider use.
"Our study also clearly emphasizes the need for the development of new analytical techniques that allow us to evaluate the formation of toxic disinfection by-products when chlorine or other disinfectants are being used,” says Prasse. “One reason regulators and utilities are not monitoring these compounds is that they don't have the tools to find them.”
The research was published in the journal Environmental Science & Technology.
Source: Johns Hopkins University via Phys.org





