Cancer is a crafty foe, not least because traces often remain after surgery and can trigger a relapse later on. Now a new technology to fight those dangerous leftovers – a cold atmospheric plasma system – has been approved for human clinical trials by the US Food and Drug Administration (FDA).
While plasma is arguably the state of matter most of us are the least familiar with, it's thought to be the most common form of ordinary matter in the universe. Most of the time it's extremely hot – just look at stars, for example – but when made for use here on Earth it can be created at low temperature by partly ionizing a gas like helium.
This kind of cold plasma has been put to work over the years killing bacteria on medical equipment, food, human skin and wounds, as well as treating lice and even eliminating the smell of deep fryers.
But one major application that's been in the works for a long time is treating cancer. In tests on animals and cultured cells, cold plasma has been found to be an effective – and selective – cancer-killer. The plasma appears to create toxic molecules called reactive oxygen species (ROS), which damage tumors but leave healthy cells alone. That's because cancer cells are already highly oxidized, so the ROS elevate that beyond safe levels and kill the cells.
And that's where a new device comes in. A prototype was developed over the past few years by researchers from US Medical Innovations LLC and the Jerome Canady Research Institute for Advanced Biological and Technological Sciences (JCRI/ABTS).
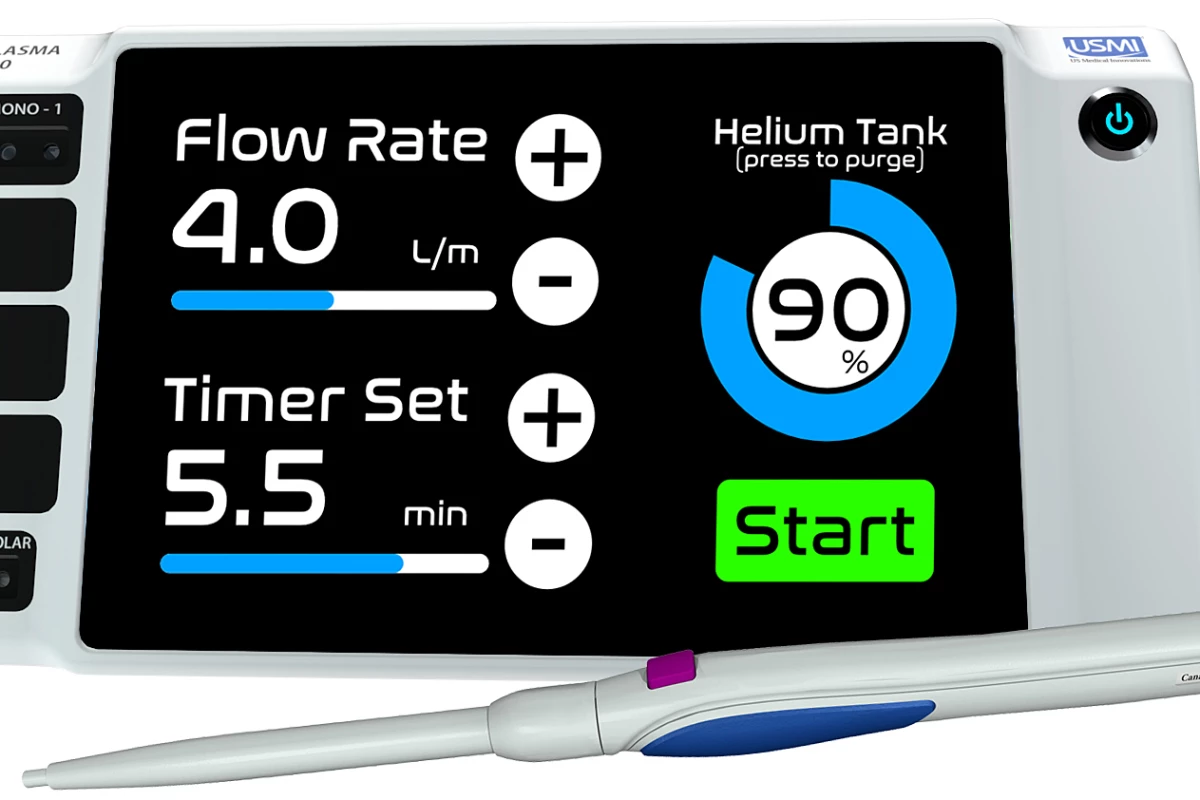
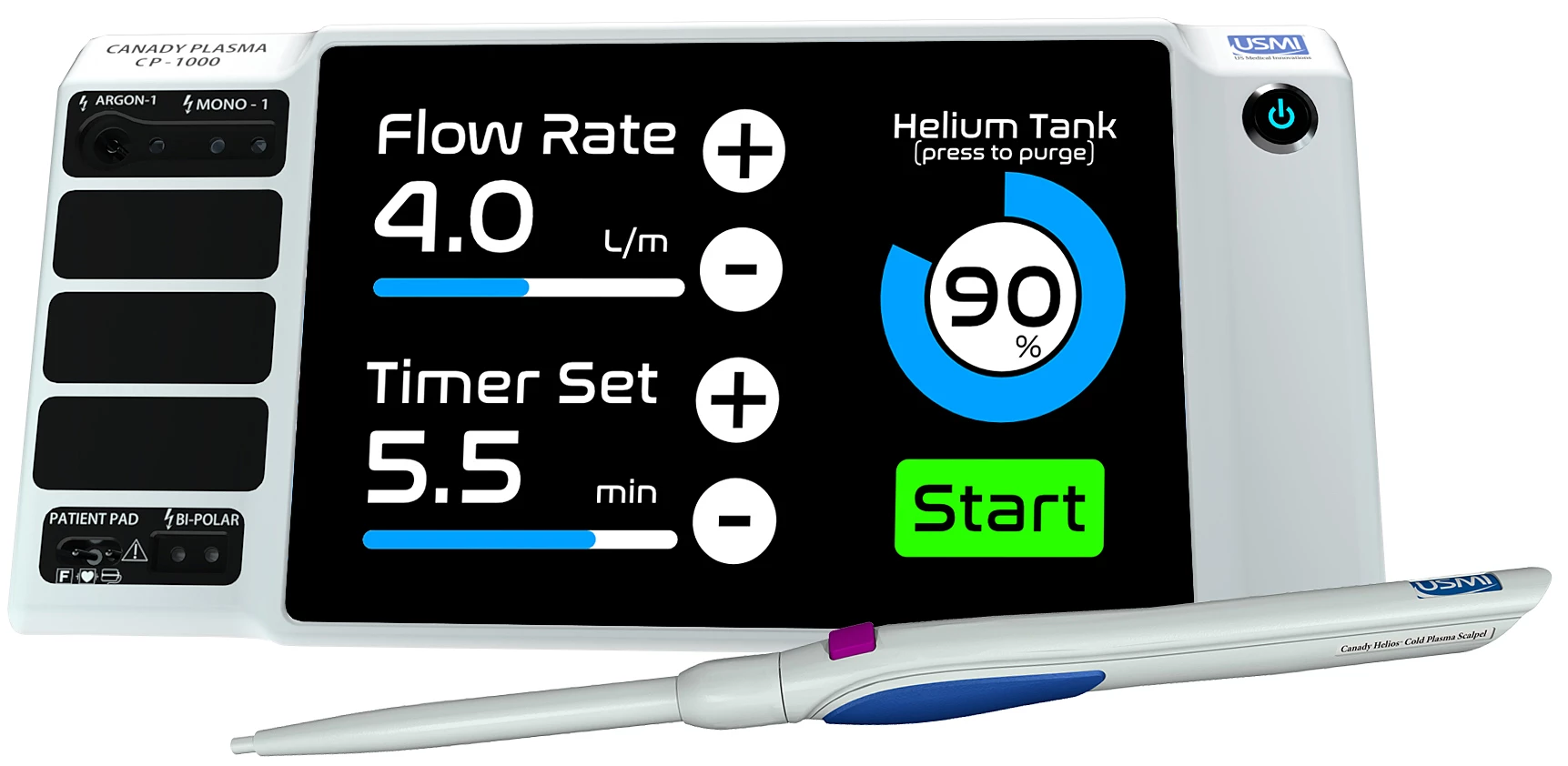
This device is called the Canady Helios Cold Plasma System and Scalpel. It's made up of a cold plasma generator connected to an electrosurgical scalpel shaped like a pen, which sprays a blue jet of cold plasma from the tip. Previous tests have shown that exposing cancer cells to this jet for between two and seven minutes is effective at killing them, without harming healthy cells.
And now the technology has leaped over the next major hurdle. Having previously been used in FDA-approved compassionate use cases, the FDA has now approved the device for use in a phase I clinical trial of 20 patients.
Cold plasma won't necessarily replace existing methods of fighting cancer, but it could join the ranks as a way to clean up residual cells that other techniques may miss. If ignored, these cells can and often do return with a vengeance.
"Cold plasma application is the fourth arm for the treatment of cancer, following chemotherapy, radiation and surgery," says Jerome Canady, leader of the team that developed the device. "There's no other 'magic bullet' out there for killing off residual tissue."
The clinical trials are due to begin recruiting in September.
Source: Purdue University