New research shows lymph nodes aren’t just cancer bystanders, they’re the command centers fueling immune attacks. Surgically removing them along with tumors may weaken treatment, while preserving them could supercharge it.
It’s been standard procedure to surgically remove lymph nodes as part of cancer treatment for roughly a century – for example, in the case of breast cancer, removing the breast and the lymph nodes from the armpit that’s closest to the cancer. The reason is to remove the potential for cancer spread.
But two recent studies by researchers at The Peter Doherty Institute for Infection and Immunity (Doherty Institute), a joint venture between the University of Melbourne and the Royal Melbourne Hospital, have demonstrated why preserving lymph nodes could improve patient outcomes and make immunotherapies more effective.
“Lymph nodes aren’t just passive waiting rooms for immune cells, they actively train and educate T cells, and send them off to do their job,” said Professor Axel Kallies, PhD, Laboratory Head at the Doherty Institute and the corresponding author on both studies. “Our research suggests that removing lymph nodes during cancer surgery, a common practice to prevent tumor spread, may inadvertently reduce the effectiveness of treatments, such as checkpoint blockade and CAR T cell therapies. Preserving lymph nodes could strengthen immune responses and increase the effectiveness of immunotherapy.”
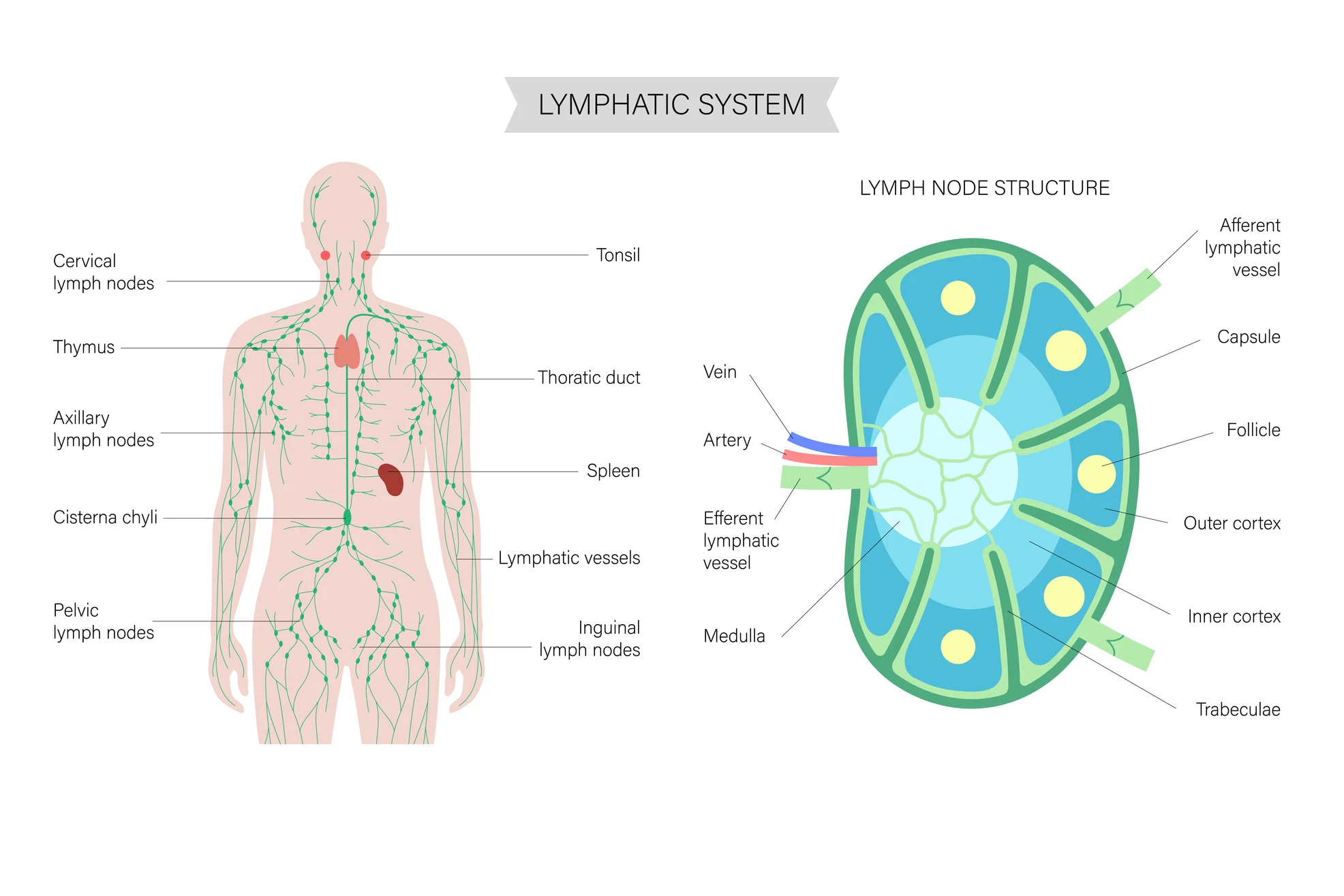
But what are lymph nodes, and what do they do? Lymph nodes are small, bean-shaped organs scattered throughout the body that act as checkpoints of the immune system. They filter lymph fluid, which drains from tissues, trapping and destroying germs, cancer cells, and other harmful material. Inside, lymph nodes are packed with immune cells like T cells, B cells, and dendritic cells that communicate, train, and multiply in response to threats.
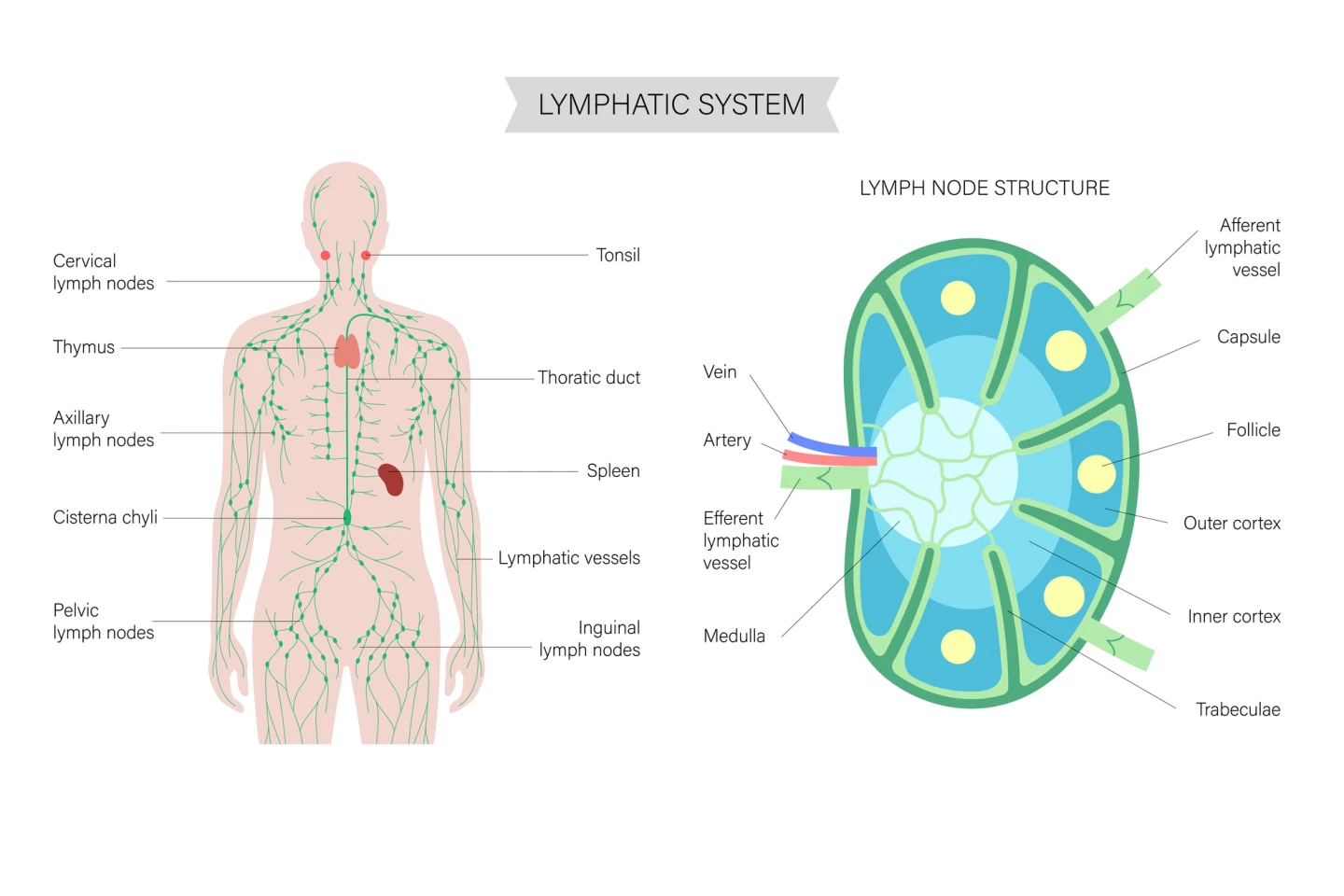
In normal health, lymph nodes are where immune responses are launched: they’re the places where incoming danger signals are processed, immune soldiers are activated, and fresh waves of defenders are sent out to fight infections or abnormal cells. So, let’s unpack what the two studies found.
Study 1
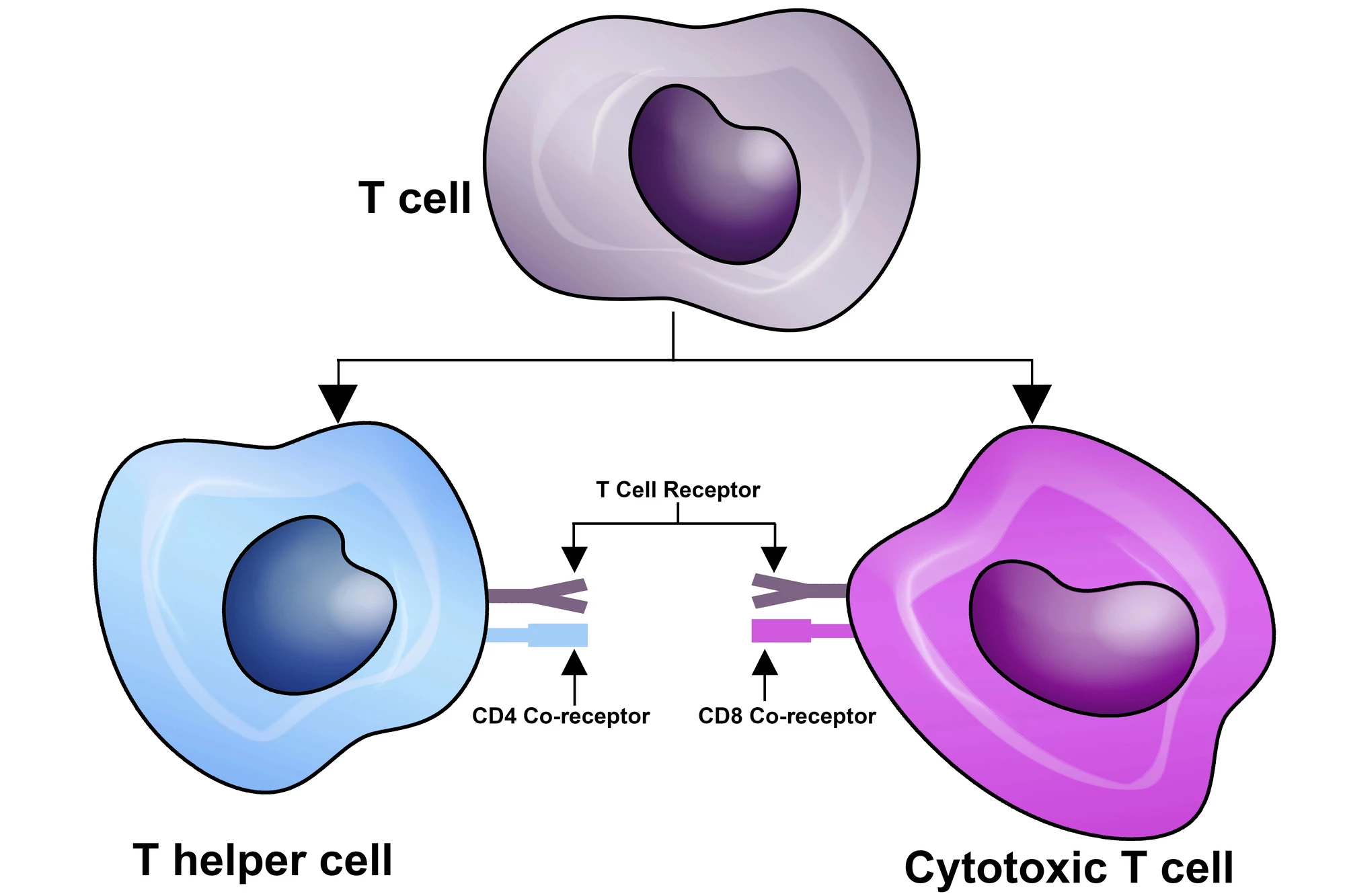
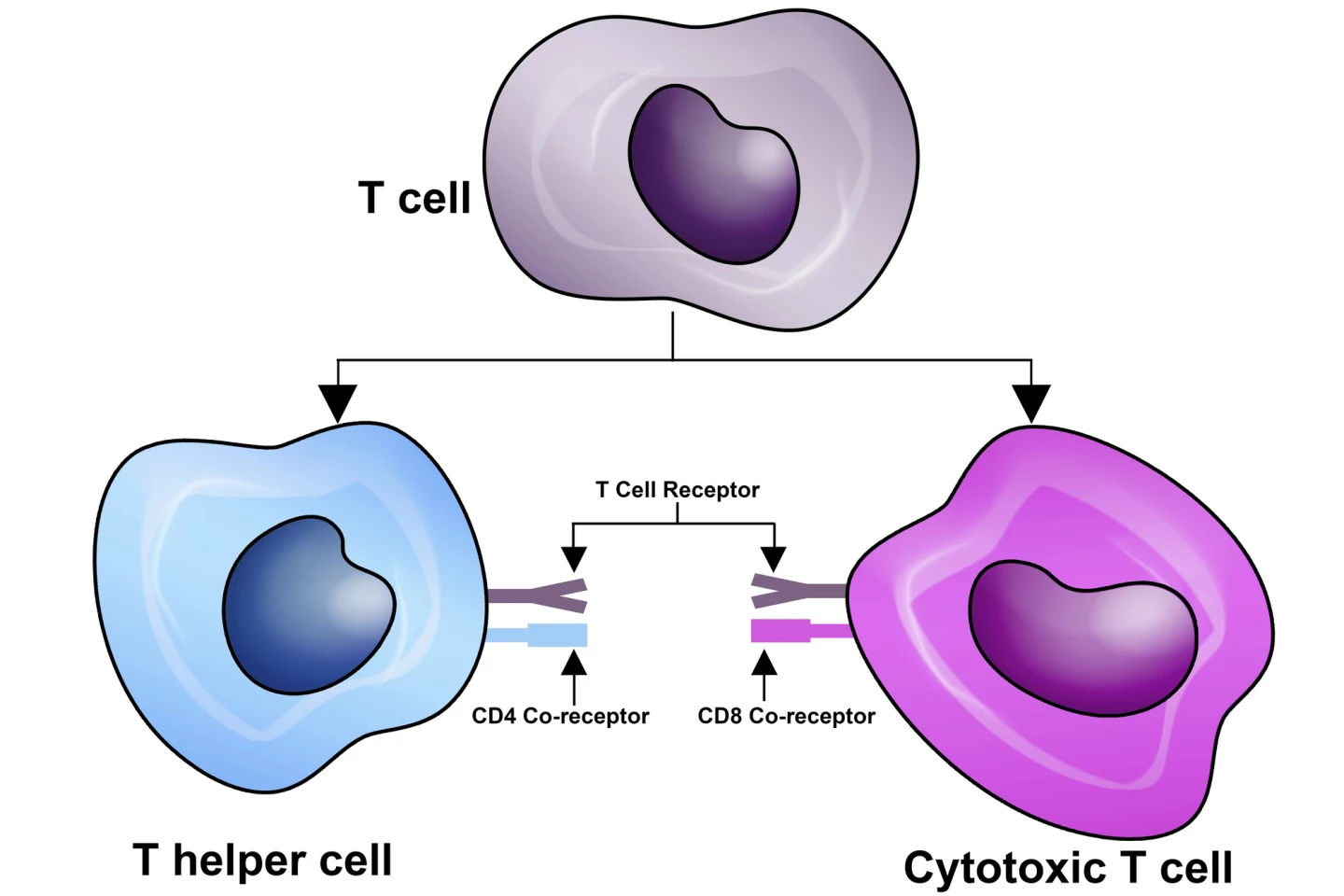
The researchers looked closely at key cancer-killing immune cells called CD8+ T cells, also known as cytotoxic T cells. CD8+ T cells work to recognize specific antigens on target cells, the “flags” of infection or cancer, then induce cell death through various mechanisms. In this study, the researchers used advanced techniques in mice and also checked human cancers. They found that inside tumors, T cells become exhausted and develop into tissue-resident cells that don’t move much and don’t contribute strongly to fighting cancer.
In contrast, special “stem-like” T cells in the draining lymph nodes, the ones connected to the tumor area, act as a supply line, sending in fresh troops of killer T cells to the tumor. These lymph node T cells are essential for responding well to immunotherapy like checkpoint blockade, which is used to block “checkpoint” proteins on immune cells and cancer cells that allow cancer cells to evade immune detection. A molecule called TGFβ forces tumor T cells into becoming “stuck” residents, which reduces the pool of useful lymph-node-based stem-like T cells.
Study 2
In this study, an extension of the first, the research team studied how T cells behave during chronic infections and during checkpoint blockage therapy. They traced how T cells develop in different immune system organs.
Lymph nodes were found to provide a special environment that nurtures stem-like T cells and helps them mature into effective “soldier” T cells. This process depends on a gene regulator called KLF2, and it’s supported by migratory dendritic cells in lymph nodes that keep presenting antigens. Without lymph nodes, this whole production line of turning stem-like T cells into effective cancer-fighting ones breaks down. During checkpoint blockade therapy, lymph nodes are where the big T cell expansion starts, fueling the body-wide immune attack.
“Our research identifies molecular signals that are involved in the regulation of stem-like cells and in their capacity to produce effective killer cells,” said Carlson Tsui, PhD, a postdoctoral researcher at the Doherty Institute and the lead author on the second study. “These findings could guide the development and refinement of immune-based treatments for cancer and chronic infection.
“Furthermore, our research shows that rather than only focusing on the tumor itself, therapies should also be designed to preserve and enhance lymph node function. By targeting these critical immune hubs, we could boost the body’s natural ability to fight cancer, increase the effectiveness of existing immunotherapies and help more patients respond to treatment.”
Together, these two studies provide a greater understanding of how lymph nodes affect immune responses. They will guide future treatment strategies, which, in turn, will improve patient outcomes.
Both studies were published in the journal Nature Immunology. Study 1 was published on 29 July 2025, and Study 2 was published on 15 September 2025.
Source: Doherty Institute