Recycling lithium-ion batteries – whether they're the large ones in your EVs or the small ones in your gadgets – is no easy task, as it requires specialized processes, high heat, and a range of chemicals to achieve. Owing to the complexity and costs involved, many used up batteries are simply sent to landfills.
To tackle this, a team of material chemists from the Massachusetts Institute of Technology (MIT) has developed a more sustainable electrolyte – the compound that facilitates the movement of lithium ions between a battery's cathode and anode. When it's added to water, it spontaneously forms a stable structure to conduct ions across its gel-like surface; when the battery is immersed into organic solvents, the electrolyte dissolves immediately, allowing for the electrolytes to be separately recycled.
That could negate the difficult processes involved in extracting more commonly used electrolytes and recycling them using expensive equipment, and prevents the production of large volumes of high-pH wastewater that can be hard to treat. In fact, it could also remove the need to use volatile, toxic, and flammable electrolytes in the first place.

Indeed, that was the idea with this work, notes Yukio Cho, who authored the paper that appeared in Nature Chemistry last week: "Our approach is to start with easily recyclable materials and figure out how to make them battery-compatible."
The researchers' new electrolyte is made of a class of molecules called aramid amphiphiles (AAs) and a compound used in petrochemical applications called polyethylene glycol (PEG). When exposed to water, the AAs form a structure of nanoribbons, which makes it tough and robust like Kevlar, a material popular in bulletproof safety equipment. The PEG, meanwhile enables the conduction of lithium ions – making for a stable electrolyte.
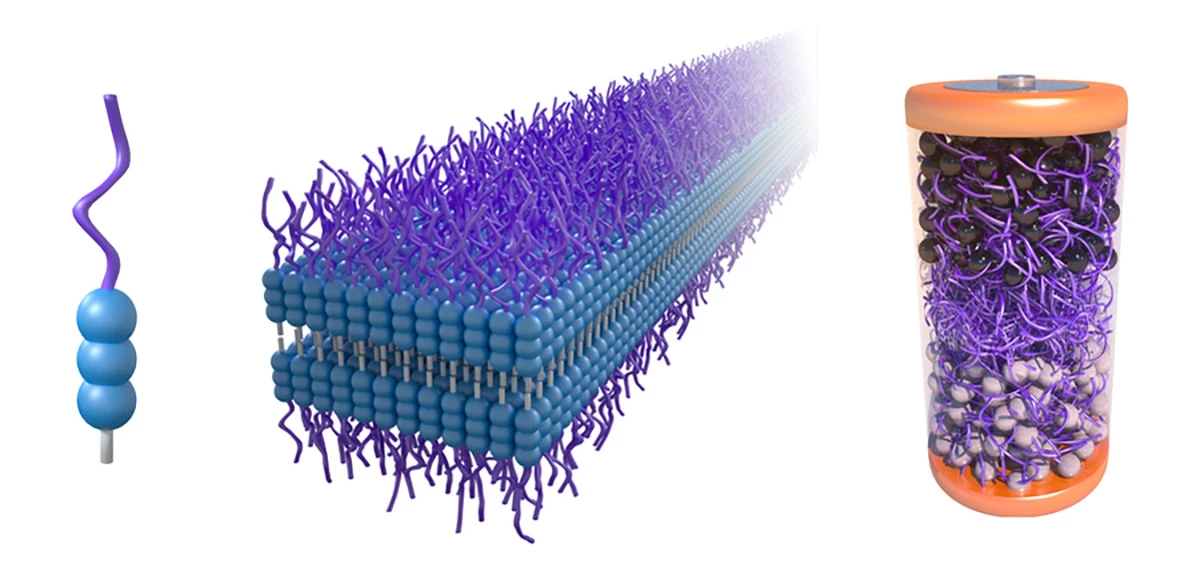
Now, remember how I said this compound "could" do great things? It turns out that while the electrolyte serves its purpose and is strong enough to endure the stresses of battery operation, it's not as performant as many commercial batteries – which means it likely wouldn't be ideal for use in EVs in its current form.
That's due to polarization, which hampers the movement of lithium ions into the battery's electrodes during rapid charging and discharging. So at this point, it's more of a proof of concept than a market-ready product.
"We don’t want to say we solved all the problems with this material," noted Cho. "Our battery performance was not fantastic because we used only this material as the entire electrolyte for the paper, but what we’re picturing is using this material as one layer in the battery electrolyte. It doesn’t have to be the entire electrolyte to kick off the recycling process,”
As such, the team is also looking at ways to integrate its Kevlar-like electrolyte into new battery chemistries.
This approach could also work alongside other ways of recycling batteries. Last year, Rice University researchers developed a way to rapidly precipitate lithium out of discarded batteries, using microwave radiation. And earlier this year, Porsche began experimenting with a three-phase process to recycle EV batteries that involved shredding used units into a granulate mixture, separating the raw materials and sorting by purity, and producing new ones from the recovered materials. Reducing our reliance on lithium could also help scale back destructive mining practices around the world.
Source: MIT News




