Biotechnology company Moderna has announced the first preliminary data from its ongoing Phase 1/2 human trial testing an mRNA vaccine designed to target four strains of influenza. The vaccine was found to be safe and effective at generating antibody responses, however, this early data suggests it may be no more effective than current flu vaccines.
Following the extraordinary success of mRNA vaccines against the sudden emergence of SARS-CoV-2 in 2020, research boomed in the field of mRNA therapeutics. Malaria, HIV and Lyme disease are just a few of the mRNA targets currently under investigation.
But the flu is perhaps the biggest target for mRNA vaccine technology. Current flu vaccines, which are reformulated every year, are generally only at best 50 or 60 percent effective at preventing infection. Not only is it hoped mRNA vaccines can increase that efficacy, but new formulations can be developed in around 60 days, meaning annual flu vaccines could be better targeted at the specific strains circulating during a given season.
While several mRNA flu vaccines are currently in development, Moderna is at the head of the pack and first to reveal interim data from its early-stage human trials. The data, announced via press release and not yet formally published, outlines the interim safety data and antibody responses from its first Phase 1 cohort.
The vaccine was found to successfully induce antibody responses against all four strains of influenza targeted. Minimal dose response differences also suggest low doses of the vaccine should be as effective as higher doses.
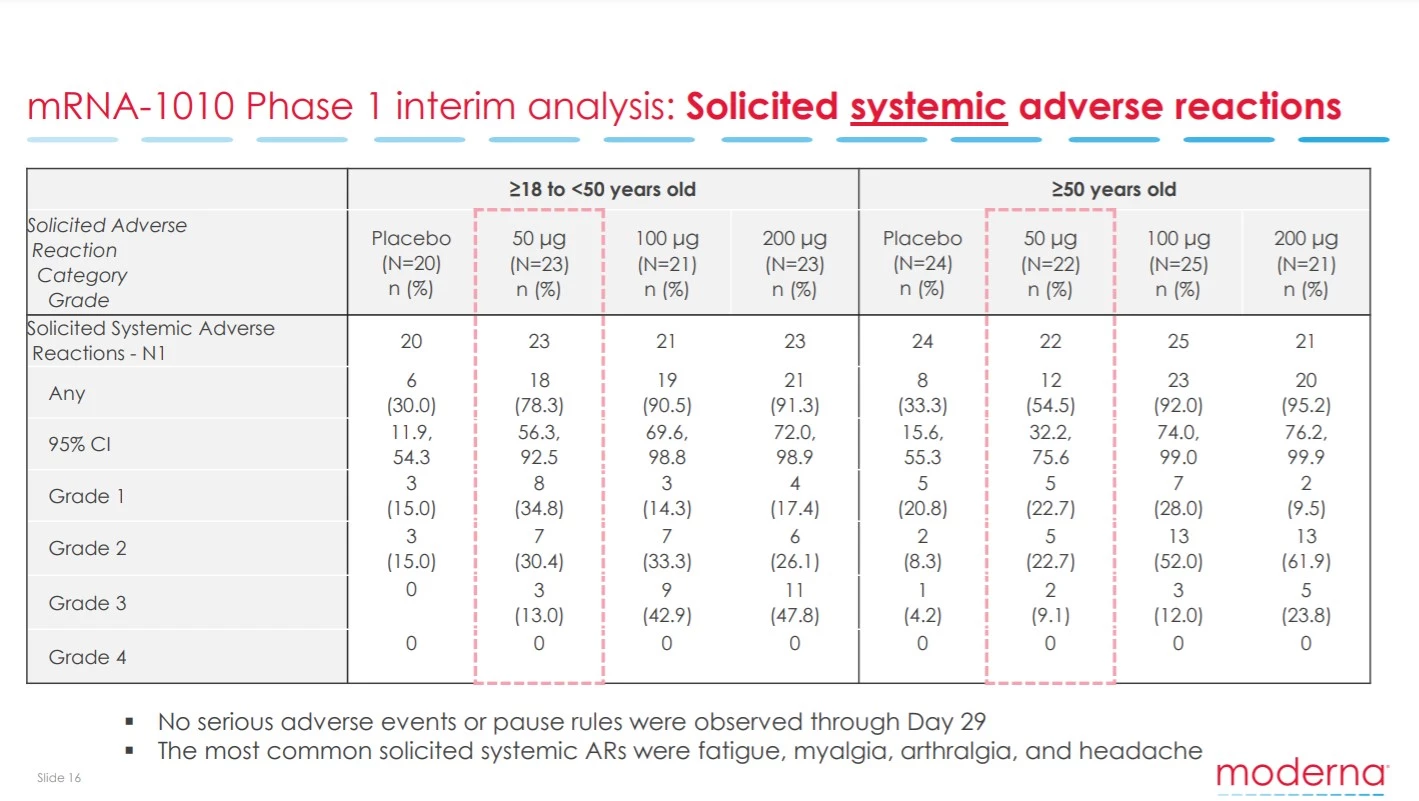
No serious adverse effects were detected in the study. However, a notable volume of Grade 2 and 3 systemic side effects were cited. These are similar to what has been seen with mRNA COVID-19 vaccines and consist of symptoms including fatigue and headache.
A Grade 3 side effect means the symptom was severe enough to interfere with one’s day-to-day life. Essentially these kind of side effects mean you are knocked out for a day or so after receiving a vaccine. These side effects were most prominent in younger cohorts but reassuringly were dose dependent. Nevertheless, the lowest vaccine dose did still result in 13 percent of those under 50 citing a Grade 3 side effect.
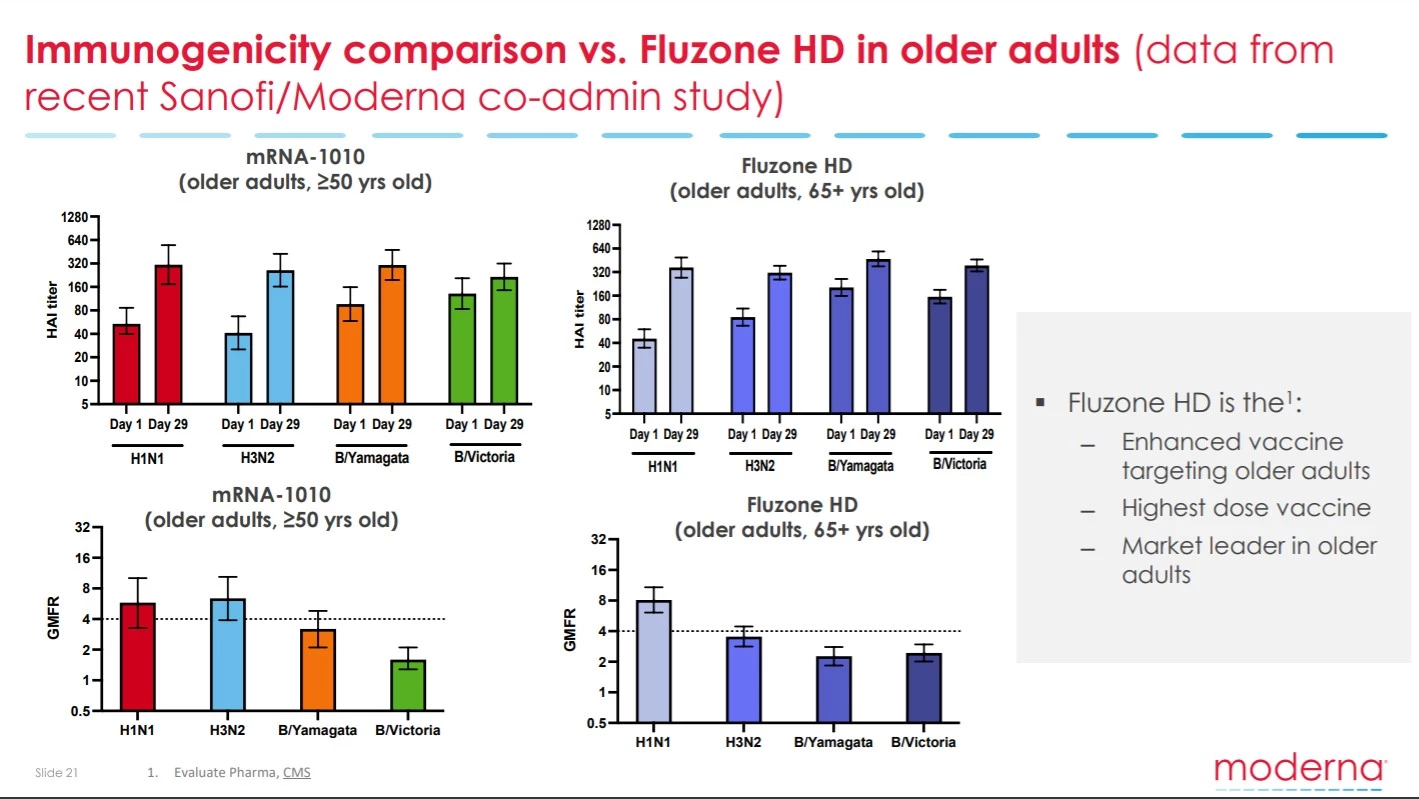
The other problematic flag in the interim data is the efficacy of this vaccine inducing antibody responses compared to what is seen with the current market-leading flu vaccine Fluzone. The data indicates Moderna’s mRNA vaccine is not much better than Fluzone.
In response to the somewhat disappointing data Moderna’s stock price plummeted by over 10 percent, indicating investors were spooked by the interim data, despite the company's attempt to put a rosy spin on the early results. The company responded by stressing this early data cannot be used as a correlate for efficacy, and Phase 2 trial data next year will offer clearer indications as to how well the vaccine performs in real-world conditions.
As chemist Derek Lowe wrote in a piece for Science, this is a reminder that mRNA isn’t magic. While mRNA vaccines can be developed faster than conventional vaccines, helping produce seasonal vaccines more swiftly, they may not be preferable to what we already have if they are just as effective but with greater acute side effects.
Stéphane Bancel, CEO of Moderna, says the overall goal for the company is to develop a single annual vaccine shot that can protect against an assortment of respiratory viruses. So instead of separate vaccines for influenza and COVID-19, the hope is mRNA technology can roll all these shots into one.
"At Moderna, our goal is to limit this suffering with an annual pan-respiratory single dose booster vaccine that is adapted to the circulating strains of SARS-CoV-2, seasonal influenza and RSV,” Bancel says.
Source: Moderna




