Researchers have made a breakthrough in cancer treatment by harnessing the disease-destroying abilities of the body’s natural killer cells. Their novel nanodrones selectively target a tumor, enabling the killer cells to do what they do best: Suppress cancer growth. The discovery opens the door to developing tumor-specific immunotherapies for hard-to-treat cancers.
Natural killer (NK) cells are white blood cells that destroy infected and diseased cells, including cancer cells, by limiting or preventing their spread. As their name suggests, they were ‘born to kill,' able to destroy potential threats without prior exposure to a particular pathogen. Now, researchers from the Ulsan National Institute of Science and Technology (UNIST), South Korea, have harnessed the power of NK cells, designing and creating nanodrones that engage the cells and direct them to target and destroy cancer.
NK cells can directly kill cancer cells by releasing cytotoxic (cell-killing) granules and cytokines that recruit other immune cells to the tumor site, enhancing the immune response against cancer cells. Because of these abilities, NK cell-based immunotherapies have been studied for a decade, including the use of NK cell engagers.
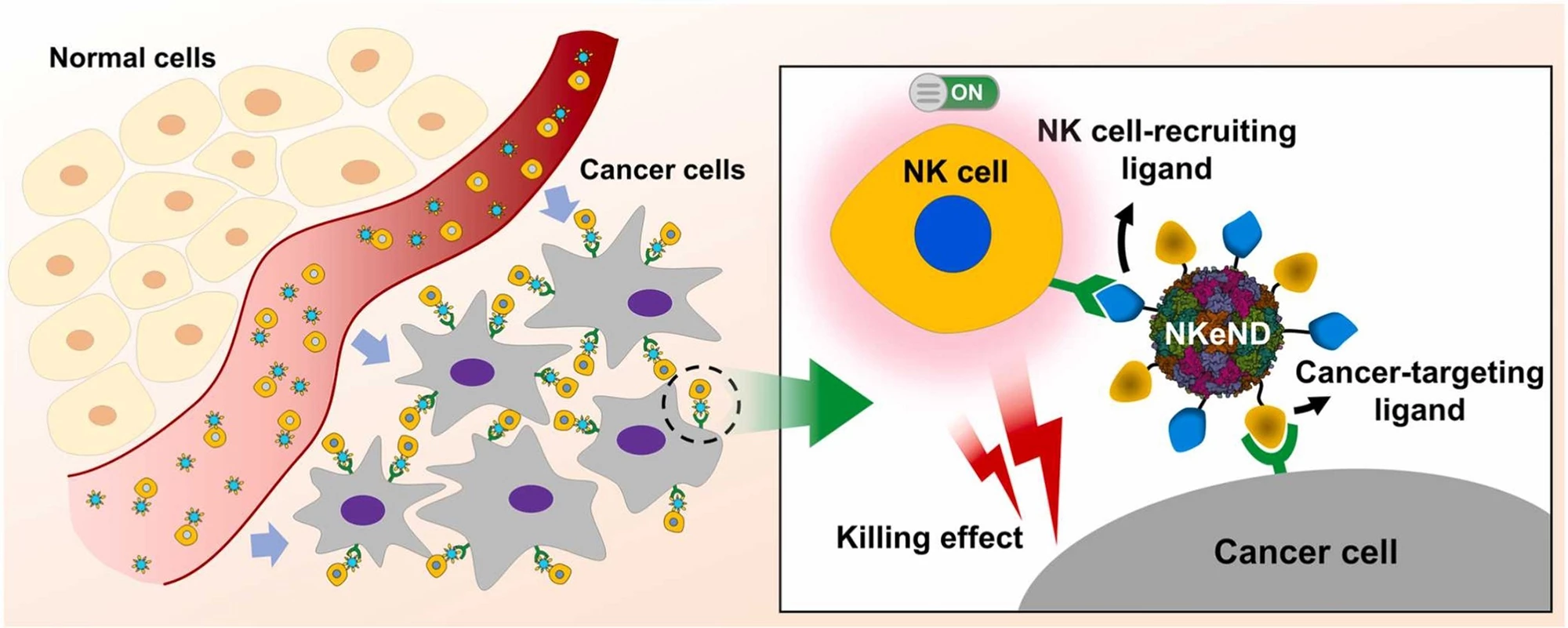
NK cell engagers are engineered proteins designed to selectively bind to both NK cells and target cancer cells, placing them in close proximity and promoting NK-mediated destruction of cancer cells. In the current study, the researchers created a nanoscale ‘protein cage’ isolated from the bacterium Aquifex aeolicus (AaLS) as a platform for the targeted delivery of NK cell-engaging nanodrones (NKeNDs) that engage and activate NK cells and subsequently deliver them to specific cancer cells to kill. The AaLS cage was fused with the SpyTag (ST) protein ligation system to enable the nanoplatform to display multiple ligands on a single nanodrone.

To ensure the NK cells were delivered to cancer cells, the researchers attached a CD16-targeting nanobody and HER2- or EGFR-binding affibodies at NK cell-specific and cancer cell-specific ligands, respectively, to the surface of their AaLS-ST nanoplatform. Let’s break that down: The CD16 receptor on NK cells makes them effective mediators of antibody-dependant cellular cytotoxicity. Affibodies (Afb) are antibody mimetics, protein ligands engineered to bind to target proteins; in this case, HER2 (human epidermal growth factor 2, a protein that promotes the growth of cancer cells) and EGFR (epidermal growth factor receptor), a protein on cells that helps them grow but when the gene that produces it is mutated, cells can grow too much and cause cancer.
The researchers tested their NKeNDs on SK-OV-3 cells, a HER2-overexpressing ovarian cancer cell line, and MDA-MB-468 cells, an EGFR-overexpressing breast cancer cell line. The NKeNDs, which had been labelled with a fluorescent dye, were seen to bind to their corresponding target cancer cells and to human NK cells. The NK cells were activated in the presence of target cancer cells.
They next assessed the cytotoxicity of the NKeNDs by adjusting the ratio of NK cells to cancer cells (N:C ratio). The extent of NK cell-mediated cytotoxicity against target cancer cells was found to be dependent not only on the number of NK cells but also on the concentrations of both NKeNDs, the ones displaying HER2 (HER2@NKeND) and the ones displaying EGFR (EFGR@NKeND). Normal, non-targeted cells showed no NK cell-mediated cytotoxicity regardless of the number of NK cells applied. The researchers say these findings imply that NK cell-mediated toxicity is primarily attributed to the direct interaction between NK cells and target cancer cells mediated by NKeNDs.
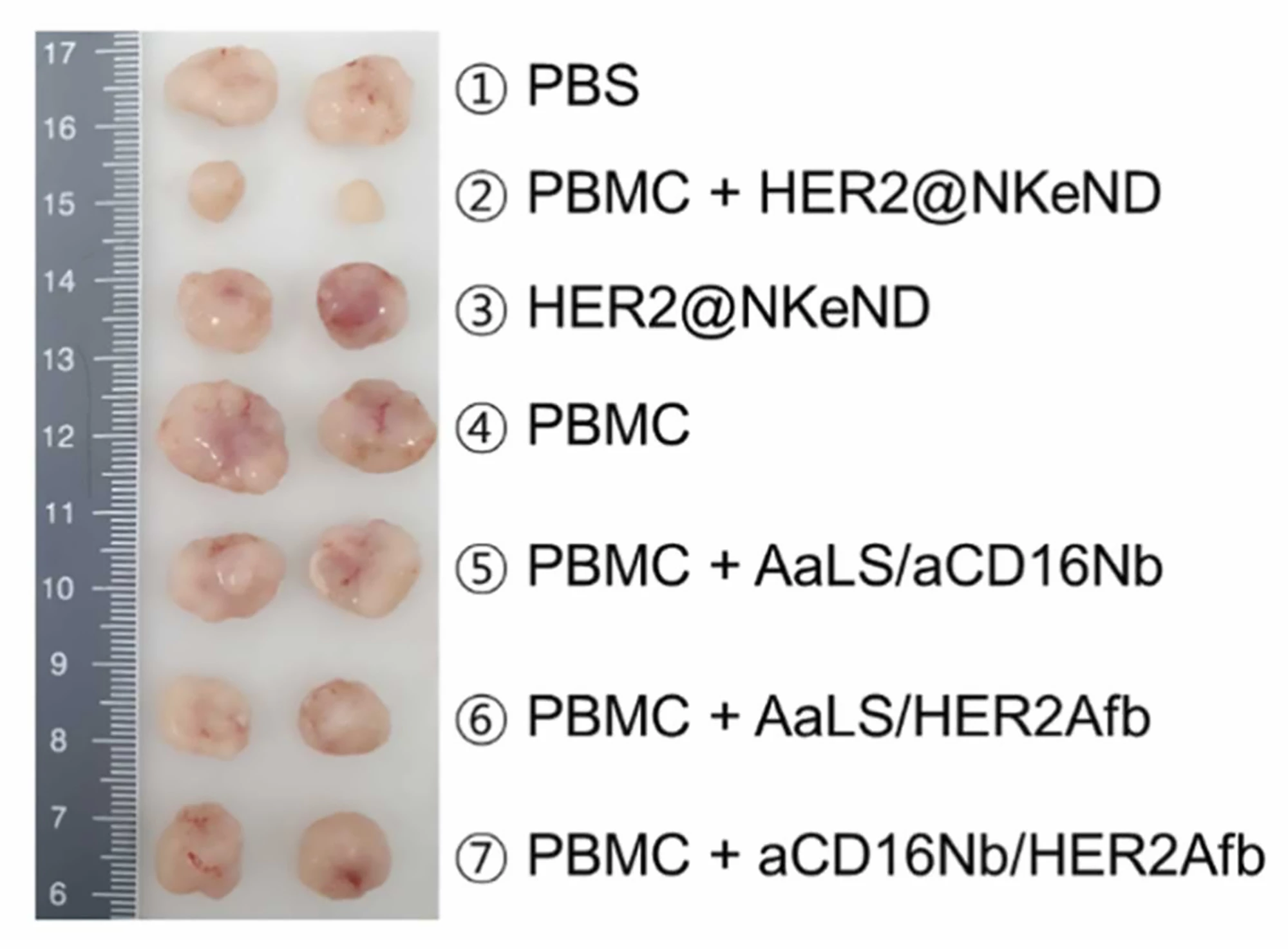
SK-OV-3 tumor-bearing mice were given intravenous injections of HER2@NKeND along with human peripheral blood mononuclear cells (PBMCs), which are any blood cells with a round nucleus, including NK cells. The researchers found that while administering HER2@NKeND alone did not suppress tumor growth, the co-administration of PBMCs and HER2@NKeND did, suggesting that the presence of PBMCs was essential for effective suppression. When they analyzed the animals histologically, the researchers found that the combination induced specific cell death within the target tumor, leading to significant suppression without off-target damage to major organs. Further, a significant number of NK cells were seen to have infiltrated the tumor sites.

When NK cells were extracted from PBMCs and injected along with HER2@NKeND, tumor growth was greatly suppressed, and the number of tumor-infiltrating human leukocytes (white blood cells) and NK cells substantially increased. However, their tumor-suppressing ability was relatively lower than when whole human PBMCs were used. The researchers attribute this to the absence of other immune cells, such as T cells, and supplementary substances that support NK cells’ cytotoxic activity.
“HER2@NKeND specifically recruits NK cells to the tumor sites, leading to the suppression of tumor growth,” said the researchers. “The efficacy of tumor suppression by HER2@NKeND relies on the presence and infiltration of human NK cells, which subsequently induce the additional infiltration of human T cells. The interplay between these T cells and NK cells may contribute to the efficient suppression of tumor growth, and further studies are required to elucidate the underlying mechanisms in more detail.”
The researchers say their study opens the door to developing novel cancer-specific treatments.
“The newly developed cancer-targeting NKeNDs (HER2@NKeND and EGFR@NKeND) hold potential as effective protein-based cancer immunotherapeutics for the selective treatment of target tumors,” they said. “Our strategy holds [the] potential to be extended to treat various cancer types by simply switching the cancer-targeting and immune cell-recruiting ligands, thereby offering new opportunities for the advancement of recombinant NK cell engagers.”
The study was published in the journal NanoToday.
Source: UNIST