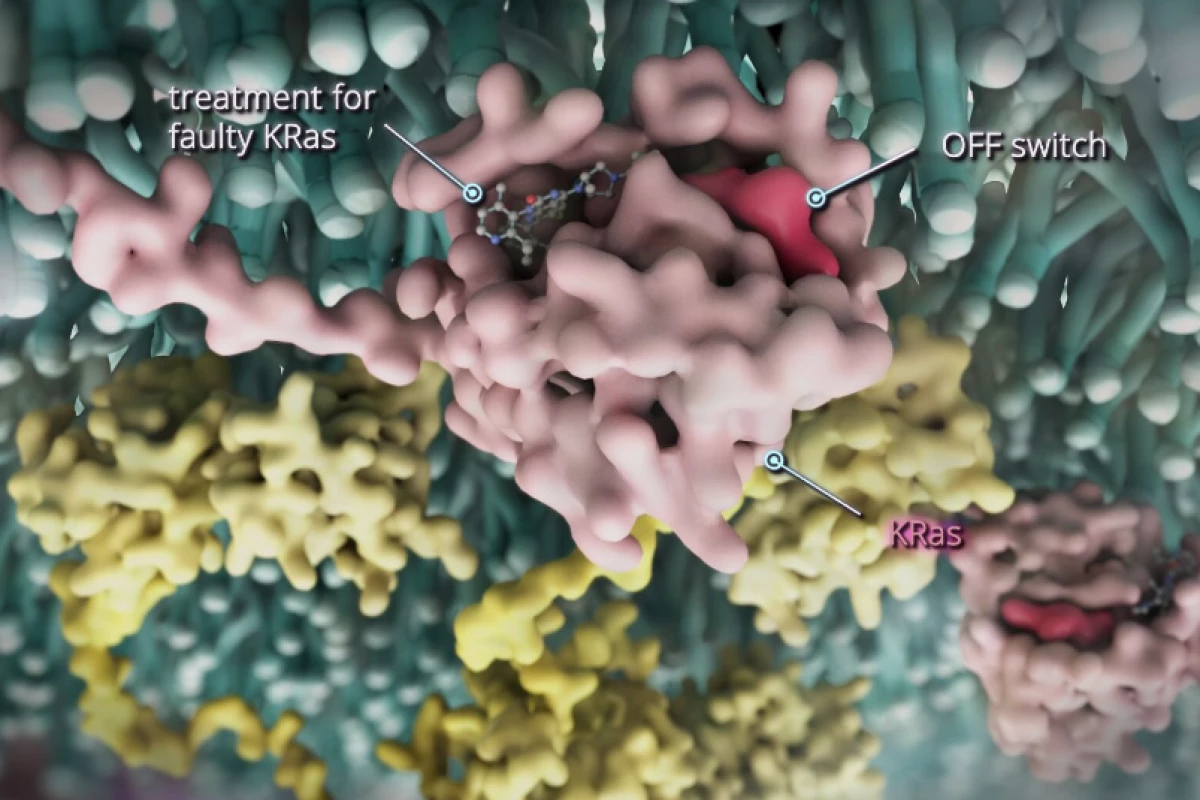
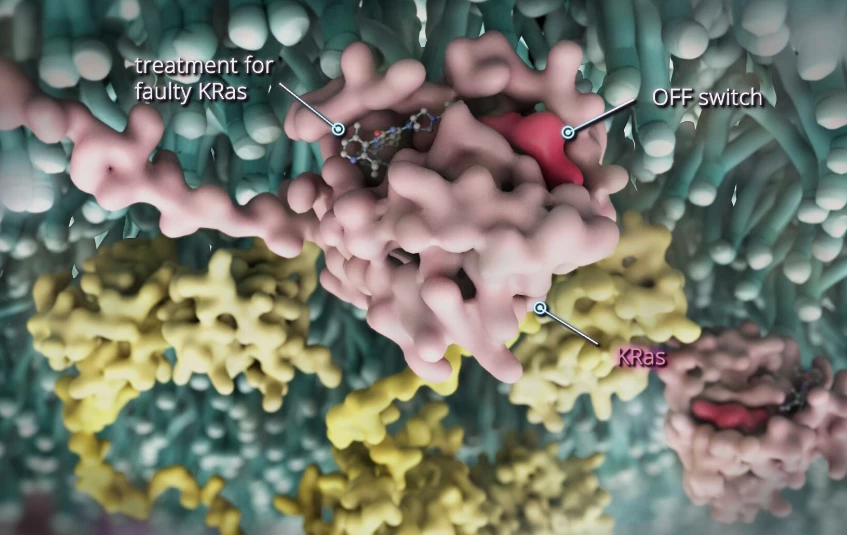
In what has already been tagged as a “game-changer” for cancer treatment, the potent once-a-day tablet known as divarasib has continued to impress at Phase 1b trial stage, outperforming not just current therapies but its previous trial results.
Following on from a standalone clinical trial earlier this year, divarasib has now been combined with the existing targeted therapy drug cetuximab, and has delivered a 62.5% positive outcome for people with advanced or metastatic colorectal cancer (CRC) linked to the KRAS G12C gene mutation.
In the first trial, at the Peter MacCallum Cancer Center in Australia, CRC patients receiving just divarasib had a 35.9% positive response rate, which was considered extremely promising.
KRAS is a key protein that regulates how cancer cells behave. For cancer patients with the KRAS G12C gene mutation, their cancer cells are far more likely to divide uncontrollably and form tumors, making the disease very challenging to treat with existing medication. As such, even though it affects around 4% of CRC patients, it has a poor prognosis.
The latest research, again led by Professor Jayesh Desai at the Peter MacCallum Cancer Center, indicates that when taken in conjunction with cetuximab, divarasib targets this mutation and is effective at slowing the development of tumors, while also being well tolerated with few adverse effects.
“The median progression-free survival for patients in the study was just over eight months and the treatment was well tolerated with manageable side effects,” Desai said. “While this is not a head-to-head trial, the response rates are better than what we have seen with other treatments that work on the KRAS G12C mutation pathway.
“We are very hopeful that this combination of divarasib with cetuximab will translate into better outcomes for our colorectal cancer patients,” he added.
While the KRAS G12C mutation is perhaps most associated with CRC, it plays a key role in the expedited progress of other cancers, such as non-small cell lung cancer (detected in around 13% of patients).
Current treatment for KRAS G12C-positive CRC patients includes 5-FU-based chemotherapy with irinotecan, oxaliplatin and/or capecitabine, however, it faces limitations due to low targeting of specific tumors and toxicity.
Earlier this year, the cancer center began a global Phase I trial of divarasib treatment on 137 cancer patients. Research found that the drug was 50 times more specific and 20 times more potent than other similar agents that are currently being used to treat the KRAS mutation.
“It has taken us years of research to build a more thorough understanding of how to target the KRAS mutation and to refine the science so we can develop molecules that are more even more potent,” Desai said in August. “This once daily tablet treatment is true precision medicine specifically targeting the genetic mutation that is driving the cancer.”
The research was published in the journal Nature Medicine.
Source: Peter MacCallum Cancer Centre via Scimex